The average speed (in meters per second) of a gas molecule is where T is the temperature
Question:
The average speed (in meters per second) of a gas molecule is
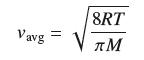
where T is the temperature (in kelvins), M is the molar mass (in kilograms per mole), and R = 8.31. Calculate dvavg/dT at T = 300 K for oxygen, which has a molar mass of 0.032 kg/mol.
Transcribed Image Text:
Vavg || 8RT ẨM
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
Using the form v av 8RTM 12 8RMT 12 ...View the full answer

Answered By

Felix Onchweri
I have enough knowledge to handle different assignments and projects in the computing world. Besides, I can handle essays in different fields such as business and history. I can also handle both short and long research issues as per the requirements of the client. I believe in early delivery of orders so that the client has enough time to go through the work before submitting it. Am indeed the best option that any client that can think about.
4.50+
5+ Reviews
19+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Mathematics questions
-
The graph shows speedometer readings, in meters per second (on the vertical axis), obtained as a skateboard travels along a straight-line path. How far does the board move between t = 3.00 s and t =...
-
Tempo Company's fixed budget (based on sales of 18,000 units) for the first quarter reveals the following. Fixed Budget Sales (18,000 units $210 per unit) $ 3,780,000 Cost of goods sold Direct...
-
The pedestrian walkway on the Golden Gate Bridge is about 75 m above the water below. This bridge is (unfortunately) a popular spot for some unhappy people, who attempt to jump off. Ignore air drag...
-
Define pricing practices of tesla INC as well as market structure.
-
When two mutually exclusive projects have different lives, how can an analyst determine which is better? What is the underlying assumption in this method?
-
'Performance appraisals are better than nothing.' Clarify what is meant by this statement.
-
What procedures are useful in verifying the adequacy of required disclosures of segment information?
-
Experiments have been conducted to determine local heat transfer coefficients for flow perpendicular to a long, isothermal bar of rectangular cross section. The bar is of width c, parallel to the...
-
With the following information below construct a future state value stream map after waste has been eliminated. Current State Value Stream Map (VSM) The current state VSM for the Import Parts...
-
Show, using the limit definition of the derivative, that (x) = |x 2 4| is not differentiable at x = 2.
-
Compute the derivative of (x) = x 1/3 using the limit definition. Multiply the numerator and denominator in the difference quotient (x + h) (x) h by /2* + /1* / 1 (y + x) + /z(y + x)
-
This short case is based on real world events at Apple and its largest supplier Hon Hai, with disguised names and changed places. It presents a fairly straightforward opportunity to address some of...
-
Image transcription text HORDES IFE Aug TO Add collections from cubaining 36,803 31,800 Aug TO Add collections from cubainng NEto Peyman - Equipment 31,80 Additional comments: what are the...
-
Answer the following 1. The total fixes cost that must be covered at each of the games 2. The portion of the fixed cost allocated to each items 3. What his unit sales would be at the breakeven for...
-
A mass m is held by two perpendicular identical springs in space in the x-y plane and is dropped from a height zo under the influence of gravity (let's call this the "dropped 2D harmonic...
-
Apply data structures to store and process information, and write Python code for each task. Question 2a staffSalaryList is a list of elements where each element is a list of two elements - staff...
-
Apply data structures to store and process information, and solve computational problems using structured programming. The rates (in cents) for sewing alphabets onto clothing are shown in Figure...
-
Anna Garden recently opened her own basket weaving studio. She sells finished baskets in addition to the raw materials needed by customers to weave baskets of their own. Anna has put together a...
-
Aztec Furnishings makes hand-crafted furniture for sale in its retail stores. The furniture maker has recently installed a new assembly process, including a new sander and polisher. With this new...
-
Use a computer algebra system to find the exact area enclosed by the curves y = x5 - 6x3 + 4x and y = x.
-
Racing cars driven by Chris and Kelly are side by side at the start of a race. The table shows the velocities of each car (in miles per hour) during the first ten seconds of the race. Use the...
-
A cross-section of an airplane wing is shown. Measurements of the thickness of the wing, in centimeters, at 20-centimeter intervals are, 5.8, 20.3, 26.7, 29.0, 27.6, 27.3, 23.8, 20.5, 15.1, 8.7, and...
-
Using JAVA through Netbeans perform the following to create Hypotenous Calculator : The console // output should look like shown below: Operation This application calculates the length of the...
-
An antibiotic loses 4% of its effectiveness every month it is in storage. When its effectiveness is below 50% it is considered expired and must be discarded. Using a while loop, code a program that...
-
Jenan is a little girl who loves Geography, and she loves collecting information about countries. Note that each country has a name, capital, area, and no. of citizens (population). Your assignment...

Study smarter with the SolutionInn App


