Figure P 41.42 shows a few energy levels of the mercury atom. a. Make a table showing
Question:
Figure P 41.42 shows a few energy levels of the mercury atom.
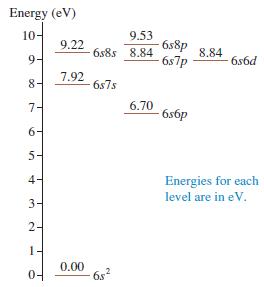
a. Make a table showing all the allowed transitions in the emission spectrum. For each transition, indicate the photon wavelength, in nm.
b. What minimum speed must an electron have to excite the 492-nm-wavelength blue emission line in the Hg spectrum?
Transcribed Image Text:
Energy (eV) 10- 9.53 6s8p 6s7p 9.22 6s8s 8.84 8.84 9- 6s6d 8- 7.92 6s7s 7- 6.70 6s6p 6- 5- 4- Energies for each level are in eV. 3- 2- 1- 04 0.00 6s
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)
Visualize Solve a We need to use the condition l 1 to ...View the full answer

Answered By

Surbhi kapoor
Finance has always been a subject of my interest. I have been indulged in helping my classmates with this subject right from the beginning of my CA cirriculum. Helping students gives me immense amount of satisfaction. Also as I have been helping my friends from a long time with this subjects of finance, accounting and cost accounting, it has developed me to make myself more expressive about the concepts of subjects. And I also feel that I understand the problem area in a particular question. This quality makes me to better understand the student's confusion and try to answer correctly.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Physics For Scientists And Engineers A Strategic Approach With Modern Physics
ISBN: 9780321740908
3rd Edition
Authors: Randall D. Knight
Question Posted:
Students also viewed these Mathematics questions
-
Figure P 41.41 shows the first few energy levels of the lithium atom. Make a table showing all the allowed transitions in the emission spectrum. For each transition, indicate a. The wavelength, in...
-
The first three energy levels of the fictitious element X were shown in FIGURE P38.56. An electron with a speed of 1.4 10 6 m/s collides with an atom of element X. Shortly afterward, the atom emits...
-
The first three energy levels of the fictitious element X are shown in FIGURE P38.56. a. What is the ionization energy of element X?b. What wavelengths are observed in the absorption spectrum of...
-
From the densities of the lines in the mass spectrum of krypton gas, the following observations were made: Somewhat more than 50% of the atoms were krypton-84. The numbers of krypton-82 and...
-
The average atmospheric pressure on earth is approximated as a function of altitude by the relation Patm = 101.325 (1 - 0.02256z)5.256, where Patm is the atmospheric pressure in kPa and z is the...
-
On January 1, 2023, Price Company acquired an 80% interest in the common stock of Smith Company on the open market for $750,000, the book value at that date. On January 1, 2024, Price Company...
-
Consider a dataset consisting of 20 observations with the following summary statistics: \(\bar{x}=0, \bar{y}=9, s_{x}=1\), and \(s_{y}=10\). You run a regression using using one variable and...
-
Liane Hansen has prepared the following list of statements about bonds. 1. Bonds are a form of interest-bearing notes payable. 2. When seeking long-term financing, an advantage of issuing bonds over...
-
Company Y , a beverage manufacturer, observed that their product's demand and supply were in perfect balance, leading to consistent sales and no surplus or shortage in the market. What does the case...
-
Market-share-analysis company Net Applications monitors and reports on Internet browser usage. According to Net Applications, in the summer of 2014, google's Chrome browser exceeded a 20% market...
-
Suppose you put five electrons into a 0.50-nm-wide one dimensional rigid box (i.e., an infinite potential well). a. Use an energy-level diagram to show the electron configuration of the ground state....
-
The ionization energy of an atom is known to be 5.5 eV. The emission spectrum of this atom contains only the four wavelengths 310.0 nm, 354.3 nm, 826.7 nm, and 1240.0 nm. Draw an energy-level diagram...
-
What are the accounts involved in recording the sale of merchandise on credit?
-
(a) A mutual fund raised Rs. 150 lakhs on April 1, 2018 by issue of 15 lakh units at Rs. 10 per unit. The fund invested in several capital market instruments to build a portfolio of Rs. 140 lakhs,...
-
(a) X Ltd. is studying the possible acquisition of Y Ltd. by way of merger. The following data are available in respect of both the companies. Particulars Market Capitalization (Rs.) Gross Profit...
-
a) Market refers to the mechanism through which all goods and services are voluntarily exchanged among different owners. Through price, markets allocate scarce resources among competing uses. Discuss...
-
(a) A company has an EPS of Rs. 2.5 for the last year and the DPS of Rs. 1. The earnings is expected to grow at 2% a year in long run. Currently it is trading at 7 times its earnings. If the required...
-
a) Risk analysis is the study of the underlying uncertainty of a given course of action and refers to the uncertainty of forecasted cash flow streams, the variance of portfolio or stock returns, the...
-
Jeff Heyl, the owner of Bascomb's Candy (Problem 1 and 2 above) realizes that he should get more information before making his final decision. He decides to contract with a market research firm to...
-
Question 2 For an n x n matrix A = form) via (aij)
-
You are given the equation used to solve a problem. For each of these, you are to a. Write a realistic problem for which this is the correct equation. b. Draw the before-and-after pictorial...
-
You are given the equation used to solve a problem. For each of these, you are to a. Write a realistic problem for which this is the correct equation. b. Draw the before-and-after pictorial...
-
A pendulum is formed from a small ball of mass m on a string of length L. As FIGURE CP10.69 shows, a peg is height h = L/3 above the pendulums lowest point. From what minimum angle θ...
-
Characterize the nature of motivation, including its importance and focus Identify and describe the need theories on motivation. Identify and describe the behavior theories on motivation. Identify...
-
Calculate the amount that Astrid should withdraw from her college savings fund of $21000 if she wishes to withdraw equal amounts at the end of each month for four years. The annual nominal interest...
-
Jason borrows $ 10300 for the next 4 years at a variable interest rate. Assume that the interest accumulates for 4 years at an annual nominal rate of 11% compounded quarterly. Calculate the...

Study smarter with the SolutionInn App


