Figure P 41.41 shows the first few energy levels of the lithium atom. Make a table showing
Question:
Figure P 41.41 shows the first few energy levels of the lithium atom. Make a table showing all the allowed transitions in the emission spectrum. For each transition, indicate
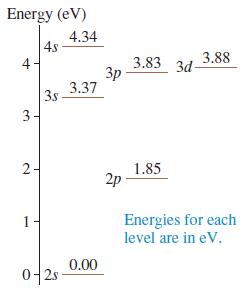
a. The wavelength, in nm.
b. Whether the transition is in the infrared, the visible, or the ultraviolet spectral region.
c. Whether or not the transition would be observed in the lithium absorption spectrum.
Transcribed Image Text:
Energy (eV) 4.34 4s 3.83 3d- 3p 3.37 3.88 4 3s 3- 2- 1.85 2p Energies for each level are in eV. 1 0.00 0-2s-
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (8 reviews)
Visualize ab and c Solve We need to use the condition l 1 ...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Physics For Scientists And Engineers A Strategic Approach With Modern Physics
ISBN: 9780321740908
3rd Edition
Authors: Randall D. Knight
Question Posted:
Students also viewed these Mathematics questions
-
Figure P 41.42 shows a few energy levels of the mercury atom. a. Make a table showing all the allowed transitions in the emission spectrum. For each transition, indicate the photon wavelength, in nm....
-
Make a table showing comparison b/w physical and chemical durability of concrete.
-
Make a table showing the sign conventions for mirrors and lenses. Include the sign convention for the mirrors and lenses themselves and for the image and object heights and distances for each.
-
A particular leadcadmium alloy is 8.0% cadmium by mass. What mass of this alloy, in grams, must you weigh out to obtain a sample containing 7.25 x 10 23 Cd atoms?
-
Repeat Prob. 1-112 for a pressure gage reading of 180 kPa.
-
On January 1, 2025, P Company acquired a 90% interest in S Company. During 2026, S Company sold merchandise to P Company at 25% above cost in the amount (selling price) of $225,000. At the end of the...
-
Effects of an Unusual Point. You are analyzing a data set of size \(n=100\). You have just performed a regression analysis using one predictor variable and notice that the residual for the 10th...
-
Go to the books companion website and use information found there to answer the following questions related to The Coca-Cola Company and PepsiCo, Inc. (a) Compute the debt to total assets ratio and...
-
Members BC and ABC need a material that can withstand at least 1643 psi. (based on chart provided a. ???Will we be able to use 1018 steel for either members ABC, BD, or both? b. ???If not would cold...
-
Mr B aged 52 years, has earned rupees 75,00,000 out of his business. His ex-wife gifted him a car worth rupees 8 lakh. He spent a total of rupees 20 lakh during a family trip. He won a lottery of 16...
-
Suppose you put five electrons into a 0.50-nm-wide one dimensional rigid box (i.e., an infinite potential well). a. Use an energy-level diagram to show the electron configuration of the ground state....
-
The ionization energy of an atom is known to be 5.5 eV. The emission spectrum of this atom contains only the four wavelengths 310.0 nm, 354.3 nm, 826.7 nm, and 1240.0 nm. Draw an energy-level diagram...
-
The specific way we choose to satisfy a need depends on our unique history, learning experiences, and cultural environment. For example, two classmates may feel their stomachs rumble during a...
-
a) A real estate contract is a contract between parties for the purchase and sale, exchange, or other conveyance of real estate. The sale of land is governed by the laws and practices of the...
-
(a) (b) (c) Small business owners are discovering that social media marketing is quickly becoming an important method for driving business growth. While the idea of using "free tools" to drive...
-
(a) The equity share of VCC Ltd. is quoted at Rs. 210. A 3-month call option is available at a premium of Rs. 6 per share and a 3-month put option is available at a premium of Rs. 5 per share....
-
(a) NP and Co. has imported goods for US $ 7,00,000. The amount is payable after three months. The company has also exported goods for US $ 4,50,000 and this amount is receivable in two months. For...
-
(a) The following information is given for 3 companies that are identical except for their capital structure: Orange Grape Apple Total invested capital 1,00,000 1,00,000 1,00,000 Debt/assets ratio...
-
A fabric firm has received an order for cloth specified to contain at least 45 pounds of cotton and 25 pounds of silk. The cloth can be woven out of any suitable mix of two yarns A and B. They...
-
During the month, services performed for customers on account amounted to $7,500 and collections from customers in payment of their accounts totaled $6,000. At the end of the month, the Accounts...
-
Two 500 g blocks of wood are 2.0 m apart on a frictionless table. A 10 g bullet is fired at 400 m/s toward the blocks. It passes all the way through the first block, then embeds itself in the second...
-
Two 500 g blocks of wood are 2.0 m apart on a frictionless table. A 10 g bullet is fired at 400 m/s toward the blocks. It passes all the way through the first block, then embeds itself in the second...
-
A 100 g granite cube slides down a 40 frictionless ramp. At the bottom, just as it exits onto a horizontal table, it collides with a 200 g steel cube at rest. How high above the table should the...
-
On-campus corporate presentations: Sympathy for the Devil Many students complain about the high number of corporate presentations taking place both on- and off-campus. In this question, we seek to...
-
How do organizations cultivate a culture of open innovation, leveraging crowdsourcing platforms, collaborative networks, and cross-industry partnerships to harness external knowledge and drive...
-
Braeden Sim is the accountant for Sim's Internet Service. From the following information, his task is to construct a balance sheet as of April 30, 201X, in proper form. Could you help him? (Click the...

Study smarter with the SolutionInn App


