Van der Waals equation of state says that 1 mole of a confined gas satisfies the equation
Question:
Van der Waal’s equation of state says that 1 mole of a confined gas satisfies the equation
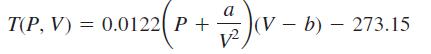
where T (ºC) is the temperature of the gas, V (cm3) is its volume, P (atmospheres) is the pressure of the gas on the walls of its container, and a and b are constants that depend on the nature of the gas.
a. Sketch the graphs of several level curves of T. These curves are called curves of constant temperature or isotherms.
b. If the confined gas is chlorine, experiments show that a = 6.49 × 106 and b = 56.2. Find T(1.13, 31.275 × 103), that is, the temperature that corresponds to 31,275 cm3 of chlorine under 1.13 atmospheres of pressure.
Step by Step Answer:

Calculus For Business, Economics And The Social And Life Sciences
ISBN: 9780073532387
11th Brief Edition
Authors: Laurence Hoffmann, Gerald Bradley, David Sobecki, Michael Price





