Ammonia gas is made industrially by the Haber process, which involves the reaction between the gases nitrogen
Question:
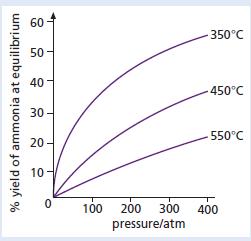
Ammonia gas is made industrially by the Haber process, which involves the reaction between the gases nitrogen and hydrogen. The amount of ammonia gas produced from this reaction is affected by both the temperature and the pressure at which the process is run. The graph shows how the amount of ammonia produced from the reaction changes with both temperature and pressure. The percentage yield of ammonia indicates the percentage of the nitrogen and hydrogen gases that are actually changed into ammonia gas.
a. Write a word and balanced chemical equation for the reversible reaction between nitrogen and hydrogen to produce ammonia using the Haber process.
b. What is meant by the term ‘reversible reaction’?
c. Use the graphs to say whether more ammonia is produced at:
(i) Higher or lower temperatures
(ii) Higher or lower pressures.
d. What is the percentage yield of ammonia if the conditions used to run the process are:
(i) A temperature of 350 °C and a pressure of 100 atmospheres?
(ii) A temperature of 550 °C and a pressure of 350 atmospheres?
e. The conditions in industry for the production of ammonia are commonly of the order of 200 atmospheres and 450 °C. What is the percentage yield of ammonia using these conditions?
f. Why does industry use the conditions stated in part e if it is possible to obtain a higher yield of ammonia using different conditions?
Step by Step Answer:





