a. State what is meant by the de Broglie wavelength of an electron. b. The diagram shows
Question:
a. State what is meant by the de Broglie wavelength of an electron.
b. The diagram shows the principles of an electron tube used to demonstrate electron diffraction.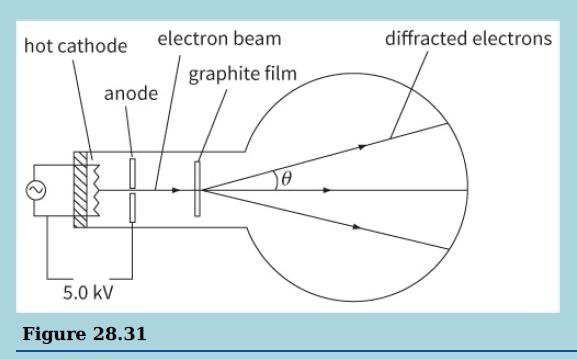
i. Calculate the kinetic energy E (in joules) of the electrons incident on the graphite film.
ii. Show that the momentum of an electron is equal to √2Eme where me is the mass of an electron, and hence calculate the momentum of an electron. (me = 9.11 × 10–31 kg)
iii. Calculate the de Broglie wavelength of the electrons.
c. Explain how the wavelengths of neutrons and electrons moving with the same energy would compare.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Cambridge International AS And A Level Physics Coursebook
ISBN: 9781108859035
3rd Edition
Authors: David Sang, Graham Jones, Gurinder Chadha, Richard Woodside
Question Posted:





