The diagram shows three of the energy levels in an isolated hydrogen atom. The lowest energy level
Question:
The diagram shows three of the energy levels in an isolated hydrogen atom. The lowest energy level is known as the ground state.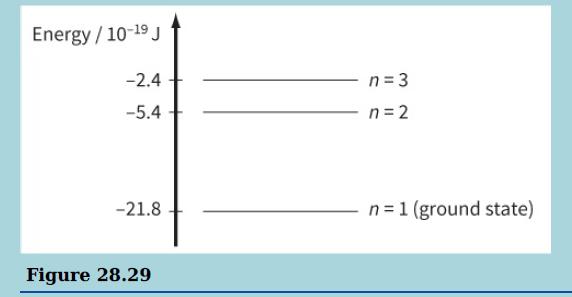
a. Explain what happens to an electron in the ground state when it absorbs the energy from a photon energy 21.8 × 10−19 J.
b. i. Explain why a photon is emitted when an electron makes a transition between energy levels n = 3 and n = 2.
ii. Calculate the wavelength of electromagnetic radiation emitted when an electron makes a jump between energy levels n = 3 and n = 2.
iii. In the diagram, each energy level is labelled with its ‘principal quantum number’ n. Use the energy level diagram to show that the energy E of an energy level is inversely proportional to n2.
Step by Step Answer:

Cambridge International AS And A Level Physics Coursebook
ISBN: 9781108859035
3rd Edition
Authors: David Sang, Graham Jones, Gurinder Chadha, Richard Woodside





