The first law of thermodynamics can be represented by the expression: U = q + W. An
Question:
The first law of thermodynamics can be represented by the expression: ΔU = q + W. An ideal gas is compressed at constant temperature. Which row shows whether ΔU, q and W are negative, positive or zero during the change?
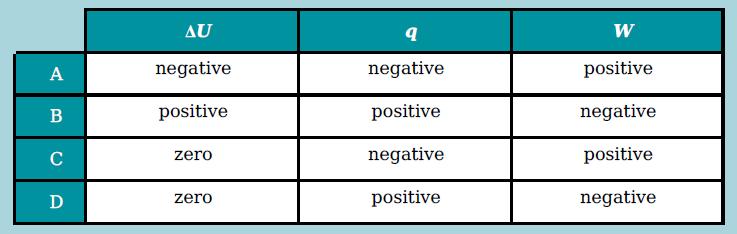
Transcribed Image Text:
AU W A negative negative positive B positive positive negative negative positive C zero positive negative D zero
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (12 reviews)
During the change of compressing an ideal gas at constant temperature U is po...View the full answer

Answered By

Akshay Shete
I have extensive experience as a tutor, both online and in-person. I have worked with students of all ages and abilities, and am skilled at adapting my teaching style to meet the needs of each individual student. I have a strong background in a variety of subjects, including math, science, and English, and am able to break down complex concepts in a way that is easy for students to understand. In addition to my subject matter expertise, I am also a patient and supportive teacher, and am committed to helping my students succeed. Whether I am working with a struggling student who needs extra help to catch up, or an advanced student looking to get ahead, I am able to provide the guidance and support they need to reach their goals. Overall, my hands-on experience as a tutor has prepared me to be a confident and effective teacher, and I am excited to use my skills to help students succeed.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Cambridge International AS And A Level Physics Coursebook
ISBN: 9781108859035
3rd Edition
Authors: David Sang, Graham Jones, Gurinder Chadha, Richard Woodside
Question Posted:
Students also viewed these Sciences questions
-
a. The first law of thermodynamics can be represented by the expression: U = q + W. State what is meant by all the symbols in this expression. b. Figure 19.18 shows a fixed mass of gas that undergoes...
-
The first law of thermodynamics says you cant really win, and the second law says you cant even break even. Explain how this statement applies to a particular device or process; alternatively, argue...
-
The first law of thermodynamics is sometimes whimsically stated as, You cant get something for nothing, and the second law as, You cant even break even. Explain how these statements could be...
-
Lucy has just been promoted to a managerial position and given a new office. She is very fond of small Persian carpets and Native American paintings and wants to get some carpets and paintings for...
-
Determine the value of a $1300 non- interest-bearing note four months before its maturity date of July 13, 2015, if money is worth 7%.
-
During its first month of operations in March 2014, Volz Cleaning, Inc., completed six transactions with the dollar effects indicated in the following schedule: Required: 1. Write a brief explanation...
-
Youve just taken a job at the central bank and are given the job of calculating the appropriate nominal interest rate for a number of different Treasury bonds with different maturity dates. You have...
-
Discuss the companys decision to grow beyond its core affluent consumer base. What did this do for the company and the brand?
-
what happens when ou use an aggregation function in a calculated column?
-
Brothers Herm and Steve Hargenrater began operations of their tool and die shop (H & H Tool) on January 1, 1987, in Meadville, PA. The annual reporting period ends December 31. Assume that the trial...
-
Explain, in terms of kinetic energy, why the temperature of a stone increases when it falls from a cliff and lands on the beach below.
-
The pendulum of a grandfather clock swings from one side to the other in 1.00 s. The amplitude of the oscillation is 12 cm. a. Calculate: i. The period of its motion ii. The frequency iii. The...
-
The graph of x 3 + y 3 = 9xy is the folium of Descartes shown in Fig. 4. (a) Find dy/dx by implicit differentiation. (b) Find the slope of the curve at (2, 4). x + y + 1 = 0 Asymptote y -X x + y =...
-
After downloading the closing price for the last 5 days of Apple stock (Ticker: AAPL) into "AAPL_file.txt", you notice "---" between each line. File Contents: 147.02 146.78 151.00 151.81 149.44 Your...
-
3) Skylar and Skyler are roommates with identical incomes (and similar names). They have $150 to spend on cellphone data (X) and economics books (Y). Their current cellphone provider, Spotty Mobile,...
-
Assume a simultaneous open market purchase of 100 million from the Bank of England and a repayment of a discount loan of 5 million from Bank A to the Bank of England. Show the overall change in their...
-
Nina has been with Elora Enterprises for 5 years and is a Customer Service Representative. She earns $2000.00 bi-weekly working 35 hours per week. She does not work any overtime. She has not reached...
-
If your car gets 25 miles per gallon, how much does it cost to drive 420 miles when gasoline costs $2.50 per gallon? The cost is $ (Simplify your answer. Round to the nearest cent as needed.)
-
The flux through a closed surface is zero. Is the electric field necessarily zero? Is the net charge inside the surface necessarily zero? Explain your answers.
-
Fahrad Inc. sells all of its product on account. Fahrad has the following accounts receivable payment experience: Percent paid in the month of sale .........10 Percent paid in the month after the...
-
Predict the major product for each of the following transformations: (a)
-
Identify the reagents that you would use to accomplish each of the following transformations (you will also need to use reactions from previous chapters). (a) (b) (c) Br Br OH
-
Predict the major product obtained when each of the following compounds is treated with bromine (Br 2 ) together with sodium hydroxide (NaOH) followed by aqueous acid (H 3 O + ). (a) (b) (c)
-
(a) (12 points) Find all numbers c in the interval (3, 4) so that the tangent line to the graph of f(x)=-3+x-3 at x = c is parallel to the line through (3, (3)) and (4, (4)).
-
a) Simplify the expression below, where u, v, and w denote suitable positive realy numbers. uv W uw V log +log +log- VW - log(uvw) Blooms Designation Score EV Major Topic Logarithm b) In a geometric...
-
8 The cost of producing a particular toaster is $(250+ 1.2n), where n is the number produced each day. If the toasters are sold for $60 each: a write an expression for the profit, P dollars b find...

Study smarter with the SolutionInn App


