a. Calculate the value of Ka for the following acids: i. 0.0200 mol dm 3 2-aminobenzoic acid,
Question:
a. Calculate the value of Ka for the following acids:
i. 0.0200 mol dm–3 2-aminobenzoic acid, which has a pH of 4.30
ii. 0.0500 mol dm–3 propanoic acid, which has a pH of 3.10
iii. 0.100 mol dm–3 2-nitrophenol, which has a pH of 4.10
b. Calculate pKa values for each of the acids in part a.
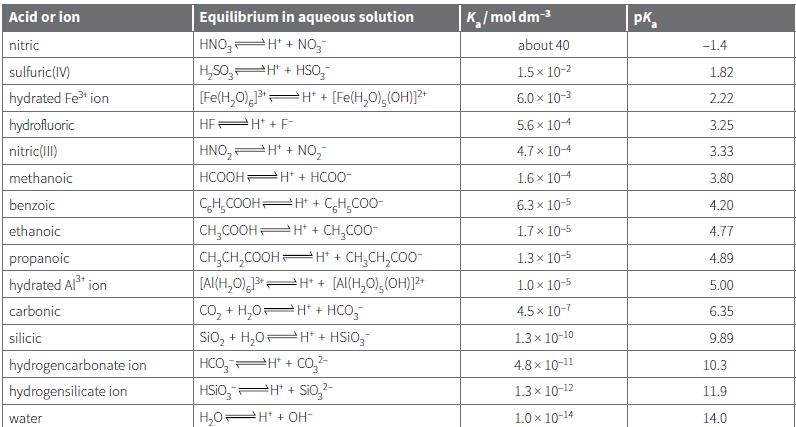
Transcribed Image Text:
Acid or ion Equilibrium in aqueous solution K/moldm-3 pK, nitric HNO, H' + NO,- about 40 -1.4 H,S0, H" + HSo, [Fe(H,0), H" + sulfuric(IV) 1.5 x 10-2 1.82 hydrated Fe ion [Fe(H,O),(OH))?* 6.0 x 10-3 2.22 hydrofluoric HEPH + F- 5.6 x 104 3.25 nitric(1II) HNO, H* + NO, 4.7 x 104 3.33 methanoic HCOOH Ht + HCO0- 1.6 x 104 3.80 CH,COOHH* + CH,CO0- CH,COOH H* + CH,CO0- benzoic 6.3 x 10-5 4.20 ethanoic 1.7 x 10-5 4.77 propanoic CH,CH,COOH H* + CH,CH,COO- 1.3 x 10-5 4.89 [Al(H,0) H* + [Al(H,0),(OH)]? Co, + H,0H* + HCO,- Sio, + H,0 H* + HSIO,- HCO, H + Co,2- hydrated Al* ion 1.0 x 10-5 5.00 carbonic 4.5 x 10-7 6.35 silicic 1.3 x 10-10 9.89 hydrogencarbonate ion 4.8 x 10-11 10.3 hydrogensilicate ion HSIO,PH + Sio,- 1.3x 10-12 11.9 water H,0H* + OH- 1.0 x 10-14 14.0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 45% (11 reviews)
a To calculate the value of Ka for each of the acids i 2aminobenzoic acid Ka HAHA The pH of the so...View the full answer

Answered By

FELIX NYAMBWOGI
I have been tutoring for over 5 years, both in person and online. I have experience tutoring a wide range of subjects, including math, science, English, and history. I have also worked with students of all ages, from elementary school to high school.
In addition, I have received training in effective tutoring strategies and techniques, such as active listening, questioning, and feedback. I am also proficient in using online tutoring platforms, such as Zoom and Google Classroom, to effectively deliver virtual lessons.
Overall, my hands-on experience and proficiency as a tutor has allowed me to effectively support and guide students in achieving their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:
Students also viewed these Sciences questions
-
The value of Ka for nitrous acid (HNO2) at 25C is given in Appendix D. (a) Write the chemical equation for the equilibrium that corresponds to Ka.
-
For the circuit in Fig. 6.70, calculate the value of R that will make the energy stored in the capacitor the same as that stored in the inductor under dc conditions. 160 uF 5A 2 4 mH
-
For the circuit in Fig. 8.68, calculate the value of R needed to have a critically damped response. 60 0.01 F 4 H
-
Calculate the pre-merger earnings per share for Admiral and Favorite and the pre-merger price-to-earnings (P/E) ratio for each company (based on the stated prices per share). Calculate the exchange...
-
Sketch the areas under the standard normal curve over the indicated intervals and find the specified areas. To the left of z = 0.72
-
During the campus Spring Fling, the bumper car amusement attraction has a problem of cars becoming disabled and in need of repair. Repair personnel can be hired at the rate of $ 20 per hour, but they...
-
Show that, in Figure 7.26, the change in potential energy along a round trip from position \(x_{1}\) to position \(x_{2}\) and then back to \(x_{1}\) is zero. Figure 7.26 (a) Cart moves directly from...
-
The yields for Treasuries with differing maturities on a recent day were as shown in the table. a. Use the information to plot a yield curve for this date. b. If the expectations hypothesis is true,...
-
1. Which of the following is a CORRECT statement? 2. 3. (a) 2.3056+10.138-7.4671 = 4.9765 (b) 2.38 x 1.0 = 2.38 8.05 (c) -=2.6 3.1 (d) (1.11 0.1) x 9.0 = 9.0 A projectile travels at 0 below...
-
Mr. Miles is a first time investor and wants to build a portfolio using only U.S. T-bills and an index fund that closely tracks the S&P 500 Index. The T-bills have a return of 5%. The S&P 500...
-
Copper(I) bromide, CuBr, is a sparingly soluble salt. (K sp = 3.2 10 8 mol 2 dm 6 ) a. What do you understand by the terms: i. Solubility product ii. Common ion effect b. Calculate the solubility of...
-
A buffer solution consists of 6.00 g of ethanoic acid (CH 3 COOH) and 12.3 g of sodium ethanoate (CH 3 COONa) in 200 cm 3 of aqueous solution. (A r values: H = 1.0, C = 12.0, O = 16.0, Na = 23.0; Ka...
-
Identify the five phases of the property division process.
-
How do feminist perspectives offer unique insights into the intersections between gender, power, and deviance, particularly concerning issues such as sexual violence and patriarchal social structures...
-
You want to be able to withdraw the specified amount periodically from a payout annuity with the given terms. Find how much the account needs to hold to make this possible. Round your answer to the...
-
What is the chemical formula of fructose? Have a look at the molecular structure. a) C5H206 b) CHO5 c) C6H2O5 d) C6HO6
-
Your portfolio which consists of 45% of stock J, and 55% of stock K. J has daily Standard Deviation 2% while K has daily Standard Deviation 3%. The correlation of two stocks is -0.5. What is the 1...
-
You are a researcher gathering data to answer the following research question: Will improving preparation methods result in higher quiz grades? You decide to interview your fellow students and ask...
-
A photon passes near a nucleus and creates an electron and a positron, each with a total energy of 8.0 MeV. What was the wavelength of the photon?
-
Parkin Industries, a U.S. company, acquired a wholly-owned subsidiary, located in Italy, at the beginning of the current year, for 200,000. The subsidiary's functional currency is the euro. The...
-
Identify the reactants that you would use to make each of the following imines: (a) (b) (c) N.
-
Predict the product of each of the following reactions: (a) (b) [1] -NH2 -H20 [H*] H2N-NH2 -H20
-
Identify the reactants that you would use to make each of the following compounds: (a) (b) NH2
-
Divide by using long division. (10x2 + 9x 2) (2x 1)
-
Convert the following to vertex form. f(x) = (x+2)(x+4) Make sure to write your answer in function form, i.e., f(x) = ...
-
Factor each expression: | 125x3 + 1 = 75-3 8a3 - 2763 =

Study smarter with the SolutionInn App


