a. The successive ionisation energies of boron are shown in Table 3.3. i. Why is there a
Question:
a. The successive ionisation energies of boron are shown in Table 3.3.
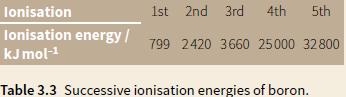
i. Why is there a large increase between the third and fourth ionisation energies?
ii. Explain how these figures confirm that the electronic structure of boron is 2, 3.
b. For the element aluminium (Z = 13), draw aB sketch graph to predict the log10 of the successive ionisation energies (y-axis) against the number of electrons removed (x-axis).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:





