Look at Figure 29.28. a. Calculate the relative molecular mass of leucine enkephalin (C 28 H 37
Question:
Look at Figure 29.28.
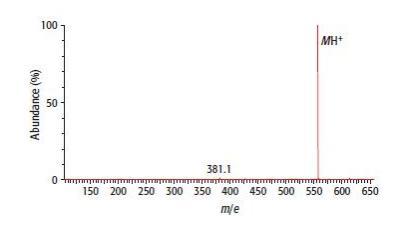
a. Calculate the relative molecular mass of leucine enkephalin (C28H37N5O7) using relative atomic masses. (Ar values C = 12.0, H = 1.0, N = 14.0, O = 16.0)
b. i. How is the peptide ionised before detection in the mass spectrometer?
ii. Why is this known as ‘soft ionisation’?
c. Why is there a peak at [MH + 1]?
d. An unexpected peak occurs at charge-to-mass ratio 578.1. This is caused by ionisation of the pentapeptide by a metal ion instead of an H+ ion. Which metal ion is responsible for this ionisation?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:





