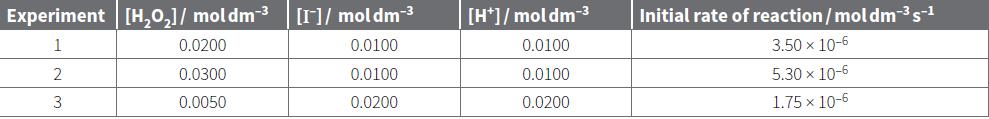
Use the data from experiments 2 and 3 in Table 22.5 to calculate the rate constant for
Question:
Use the data from experiments 2 and 3 in Table 22.5 to calculate the rate constant for the following reaction.
H2O2(aq) + 2I–(aq) + 2H+(aq) → 2H2O(l) + I2(aq)
The rate equation for this reaction is: rate of reaction = k[H2O2] [I–]
b. Use the formula t1/2 = 0.693/k to calculate a value for the rate constant of a reaction which is first order and has a half-life of 480 s.
c. A first-order reaction has a rate constant of 9.63 × 10–5 s–1. Calculate a value for the half life of this reaction.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:





