A 5-kg copper ball at 75 C is dropped into 12 kg of water, initially at
Question:
A 5-kg copper ball at 75◦C is dropped into 12 kg of water, initially at 5◦C, in a well-insulated container.
a. Find the common temperature of the water and copper ball after the passage of a long period of time.
b. What is the entropy change of the water in going from its initial to final state? Of the ball? Of the composite system of water and ball?
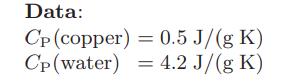
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





