A considerable amount of methane is produced in Norway from its North Sea oil and gas wells.
Question:
A considerable amount of methane is produced in Norway from its North Sea oil and gas wells. Some of this methane is converted to methanol by partial oxidation, and then biochemically converted to biomass that is used as animal feed. The reported stoichiometry for the biochemical reaction is
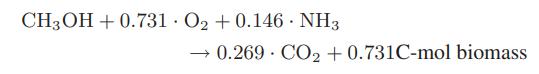
a. What is the atomic composition of the biomass produced?
b. How much heat is released per mole of methanol consumed?
c. What fraction of the Gibbs energy of the reactants is present in the biomass?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





