A gas is continuously passed through an adiabatic turbine at the rate of 2 mol/s. Its initial
Question:
A gas is continuously passed through an adiabatic turbine at the rate of 2 mol/s. Its initial temperature is 600 K, its initial pressure is 5 bar and its exiting pressure is 1 bar. Determine the maximum rate at which work can be obtained in this process. The gas is described by an augmented Clausius equation of state
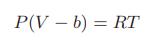
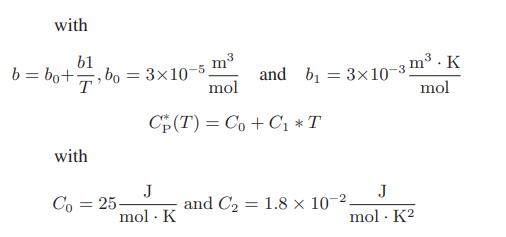
Transcribed Image Text:
P(V - b) = RT
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
To determine the maximum rate at which work can be obtained in this process we need to calculate the ...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
A gas is continuously passed through an adiabatic turbine at the rate of 2 mol/s. Its initial temperature is 600 K, its initial pressure is 5 bar and its exiting pressure is 1 bar. Determine the...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
An ideal gas expands in an adiabatic turbine from 1200 K, 600 kPa to 700 K. Determine the turbine inlet volume flow rate of the gas, in m3/s, required to produce turbine work output at the rate of...
-
Telstar Limited was incorporated on 2 January 2015. On 3 January 2015, the company ordered plant from Germany at a cost of E1 million. The plant was loaded free on board in Hamburg on 1 March 2015,...
-
John Novosel was employed by Nationwide Insurance Company for fifteen years. Novosel had been a model employee and, at the time of discharge, was a district claims manager and a candidate for the...
-
Using the Playtime Park information presented, do the following tasks. Requirements 1. Suppose Playtime Park cuts its ticket price from $60 to $54 to increase the number of tickets sold. Compute the...
-
How accurate do you regard Harvey Elliott's views to be as a general comment on current management practice? LO9
-
Financial statement data of American Traveler Magazine include the following items: Cash . . . . . . . . . . . . . . . . . . . . . . $ 23,000 Accounts receivable, net . . . . . . . 79,000 Inventories...
-
Cost Behavior, Classification Smith Concrete Company owns enough ready-mix trucks to deliver up to 145,000 cubic yards of concrete per year (considering each truck's capacity, weather, and distance...
-
On Monday, a certain stock closed at $10 per share. On Tuesday, you expect the stock to close at $9, $10, or $11 per share, with respective probabilities 0.3, 0.3, and 0.4. On Wednesday, you expect...
-
Use the Estimation tool in Aspen Plus to estimate the physical properties of methyl vinyl ketone (MKK) after entering structure using the Molecular Structure tool.
-
In a continuous manusfacturing process chlorodifluoromethane (CHClF 2 ) initially at 10 bar and 420C passes through and adiabatic pressure reducing valve so that its pressure is reduced to 0.1 bar...
-
If the technology for an industry involves high fixed capital investment, then one way to seek higher profit growth is by pursuing: A. Economies of scale. B. Diseconomies of scale. C. Removal of...
-
A licensee recently was placed on court - ordered probation. Does the licensee have to report this to the Board?
-
1. Technology and Operations What task does the operations function in a manufacturing organisation and in a service organisation perform? How does operations strategy contribute to make to corporate...
-
Do the Following current market analysis - geographic , psychographic and behavioral of Klean Kanteen THIS IS THE DETAILS AND DRAFTS OF PAPER. (THIS IS THE BASIS) Open the link;...
-
who do you think sets the underlying ethical standards when the law is fuzzy on an issue? as business and societal issues develop in the future, how does your opinion in this area inform your...
-
how do i introduce low risk high reward for a new medical assistant supervisor role in an organization?
-
Consider the DNA-derived tetranucleotide G--A--C--T. What products will be obtained when this tetranucleotide is hydrolyzed by each of the following? a. Base b. Base, followed by acid
-
The words without recourse on an indorsement means the indorser is: a. not liable for any problems associated with the instrument. b. not liable if the instrument is dishonored. c. liable personally...
-
For several values of x, use MATLAB to con rm that cosh -1 x = ln(x + x 2 - 1), x 1
-
The capacitance of two parallel conductors of length L and radius r, separated by a distance d in air, is given by where is the permittivity of air ( = 8.854 10 -12 F/m). Write a script le that...
-
When a belt is wrapped around a cylinder, the relation between the belt forces on each side of the cylinder is F 1 = F 2 e where is the angle of wrap of the belt and & is the friction coefficient....
-
OMEGA Hotel provides a type of rooms with a sale price of 50 euros. Its total fixed cost amounts to 100,000 euros. The variable cost per room was estimated at 25 euros. The dead point in rooms is:...
-
You are required to use a financial calculator or spreadsheet (Excel) to solve 10 problems (provided on page 5) on the applications of the time value of money. You are required to show the following...
-
Mongo Bongo sells $7,500 of its bongos on credit on a daily basis. Because Mongo Bongo deals with beatniks, it takes 75 days to collect its A/R. (1a) What is the average A/R that is reported on...

Study smarter with the SolutionInn App


