A proposed chemical composition for anthracite coal is C 240 H 90 O 4 NS. The stoichiometry
Question:
A proposed chemical composition for anthracite coal is C240H90O4NS. The stoichiometry for a biochemical reaction of anthracite with water is as follows:
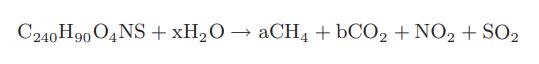 Determine the maximum thermodynamically allowed value of the extent of conversion of anthracite to methane, and is the value of a, for this reaction if no nutrients or other energy sources are provided. How many moles of carbon dioxide are produced per mole of coal (that is, the value of b)?
Determine the maximum thermodynamically allowed value of the extent of conversion of anthracite to methane, and is the value of a, for this reaction if no nutrients or other energy sources are provided. How many moles of carbon dioxide are produced per mole of coal (that is, the value of b)?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





