a. Show that the minimum amount of work, W s mi n , necessary to separate 1
Question:
a. Show that the minimum amount of work, Wsmin , necessary to separate 1 mole of a binary mixture into its pure components at constant temperature and pressure is
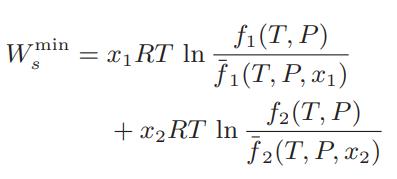
b. Show that this expression reduces to
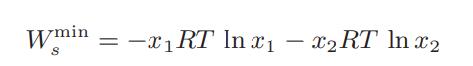
for (i) an ideal liquid mixture and (ii) a gaseous mixture for which the Lewis-Randall rule is obeyed.
c. Calculate the minimum amount of work needed to separate a 50/50 mixture of two isomers at 300 K and a pressure of 1 bar into its pure components at the same temperature and pressure. Explicitly state and justify all assumptions.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





