a. The following data have been reported for the vapor pressure of ethanol as a function of
Question:
a. The following data have been reported for the vapor pressure of ethanol as a function of temperature.
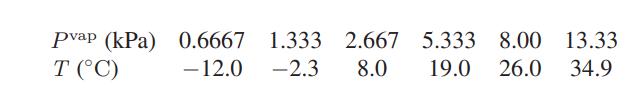 Use these data to calculate the heat of vaporization of ethanol at 17.33°C.
Use these data to calculate the heat of vaporization of ethanol at 17.33°C.
b. Ackermann and Rauh have measured the vapor pressure of liquid plutonium using a clever mass effusion technique. Some of their results are given here:
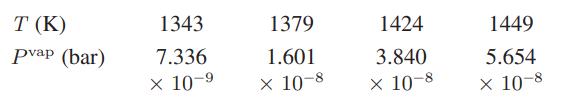
Estimate the heat of vaporization of liquid plutonium at 1400 K.
Transcribed Image Text:
Pvap (kPa) T (°C) 0.6667 1.333 2.667 5.333 8.00 13.33 - 12.0 -2.3 8.0 19.0 26.0 34.9
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
The following data have been reported for the vapor pressure of ethanol as a function of temperature ...View the full answer

Answered By

Mishark muli
Having any assignments and any other research related work? worry less for I am ready to help you with any task. I am quality oriented and dedicated always to produce good and presentable work for the client once he/she entrusts me with their work. i guarantee also non plagiarized work and well researched work to give you straight As in all your units.Feel free to consult me for any help and you will never regret
4.70+
11+ Reviews
37+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
A spring with a torsional stiffness k is attached to the hinge at B. It is unstretched when the rod assembly is in the vertical position. Determine the weight W of the block that results in neutral...
-
Problem 3 (Required, 25 marks) We consider a 5-year straight term bond issued today. The bond pays coupon quarterly and the current annual effective yield rate is i = 7.1859%. You are also given that...
-
The following data have been reported for a sample of 10 major U.S. zoological parks: a. Determine the least-squares multiple regression equation. b. Interpret the y-intercept and partial regression...
-
A manager found the following information for his company: Profit margin = 0.08 and total asset turnover = 1.85. Given that the total assets of the company is $200 million, the total liabilities is...
-
Intercontinental Widgets, Inc., had applied for a patent for a new state-of-the-art widget, which, if patented, would significantly increase the value of Inter-continentals shares. On September 1,...
-
\(C_{P}=C_{V}\) for a fluid which is (a) Incompressible (b) Compressible (c) Of very low volume expansivity (d) None of these.
-
Lindstedt's perturbation method gives a. periodic and nonperiodic solutions b. periodic solutions only c. nonperiodic solutions only
-
Pro-Weave manufactures stadium blankets by passing the products through a weaving department and a sewing department. The following information is available regarding its June inventories: The...
-
b. A bank sells a three against six $4,000,000 FRA for athree-month period beginning three months from today and ending sixmonths from today. The purpose of the FRA is to cover the interestrate risk...
-
Koto Merchants uses a perpetual inventory system and both an accounts receivable and an accounts payable subsidiary ledger. Balances related to both the general ledger and the subsidiary ledgers for...
-
a. Derive Eq. 7.4-8. b. Derive Eq. 7.4-12. c. Obtain an expression for the fugacity of a pure species that obeys the van der Waals equation of state in terms of Z, B = P b/RT, and A = aP/(RT) 2...
-
The following data are available for water: a. Compute the triple-point temperature and pressure of water. b. Compute the heat of vaporization, the heat of sublimation, and the heat of fusion of...
-
Some programming languagesfor example, Pascalhave used the semicolon to separate statements, while Java uses it to terminate statements. Which of these, in your opinion, is most natural and least...
-
You are now planning to establish ABC Company which requires total investment of $8 million. Assume interest rate on borrowing (before tax) is 3%, cost of equity is 8% and tax rate is 30%. ('1)...
-
You want to buy a $1,000,000 house. Suppose you make a 20% down payment today, and you finance the rest of your purchase with a 30-year fixed 4% interest rate.. How long will it take you to reduce...
-
On July 1, 2009, a U.S. company enters into a forward contract to buy 1 million Swiss Francs on January 1, 2010; the forward price is $0.95/SF. On September 1, 2009, it enters into a forward contract...
-
Q.3 A co. is considering two mutually exclusive projects. Both require an initial cash outlay of Rs. 10,000 each for machinery and have a life of 5 yrs. The co. required rate of return is 10% and...
-
A portfolio has an assigned beta of 1.05, the risk-free rate is 2.5%, and the market rate of return is 8.0%. What is the project's expected rate of return? Analyse whether the investor should invest...
-
A security analyst calculates the following ratios for two banks. How should the analyst evaluate the financial health of the twobanks? Bank A Bank B Return on equity Return on assets Equity...
-
9.Consider the reaction 3NO2(g)+H2O=2HNO3(aq)+NO(g) where Delta H=-137 kJ.How many kilojoules are released when 92.3g of NO2 reacts?
-
Write a MATLAB assignment statement for each of the following functions, assuming that w, x, y, and z are row vectors of equal length and that c and d are scalars.
-
a. After a dose, the concentration of medication in the blood declines due to metabolic processes. The half-life of a medication is the time required after an initial dosage for the concentration to...
-
A cable of length Lc supports a beam of length Lb, so that it is horizontal when the weight W is attached at the beam end. The principles of statics can be used to show that the tension force T in...
-
Fluid mechanics NUMERICAL PROBLEMS (4 X 3 = 12 Points) Q1. An oil flows through 50.0 m of cast iron pipe having a diameter of 0.25 m. If the volumetric flow is 0.40 m^3/s, determine the head loss in...
-
Sam Green was riding his bicycle along a road that was under construction; although he was riding against traffic instead of with traffic as required by law, he was off the pavement at the time...
-
The decision by Attorney General Jeff Sessions to revive some of the toughest practices of the "war on drugs" can have both positive and negative impacts on the correctional system and sustainable...

Study smarter with the SolutionInn App


