Compute the difference between the pure-component and partial molar enthalpies for both components at 298.15 K and
Question:
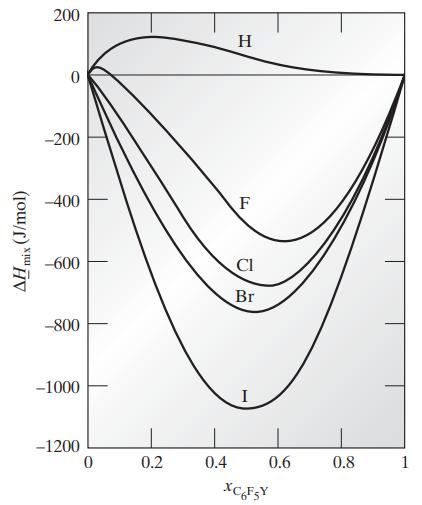
Compute the difference between the pure-component and partial molar enthalpies for both components at 298.15 K and various compositions in each of the following mixtures using the data in Fig. 8.1-2b.
a. Benzene–C6F5H
b. Benzene–C6F6
c. Benzene–C6F5Cl
d. Benzene–C6F5Br
e. Benzene–C6F5I
Fig. 8.1-2b
Transcribed Image Text:
(J/mol) mix ΔΗ, 200 -200 -400 -600 -800 -1000 -1200 0 0.2 0.4 H F Cl Br 0.6 XC6FsY 0.8 1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
Tocompute the difference between the purecomponent and partial molar enthalpies for both components at 29815 K and various compositions in each of the ...View the full answer

Answered By

Anjali Arora
Having the experience of 16 years in providing the best solutions with a proven track record of technical contribution and appreciated for leadership in enhancing team productivity, deliverable quality, and customer satisfaction. Expertise in providing the solution in Computer Science, Management, Accounting, English, Statistics, and Maths.
Also, do website designing and Programming.
Having 7 yrs of Project Management experience.
100% satisfactory answers.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
The Hampshire Company manufactures umbrellas that sell for $12.50 each. In 2014, the company made and sold 60,000 umbrellas. The company had fixed manufacturing costs of $216,000. It also had fixed...
-
The heat-of-mixing data of Featherstone and Dickinson [J. Chem. Thermodyn., 9, 75 (1977)] for the n-octanol + n-decane liquid mixture at atmospheric pressure is approximately fit by with T in K and x...
-
The U.S. Office of Management and Budget (OMB) requires government agencies to produce annual performance and accounting reports (PARS) each year. A research team at George Mason University evaluated...
-
Giroud plc is considering two alternative investment opportunities. Each of the two projects has an expected life of five years and requires an initial investment of $100,000. A feasibility study...
-
On September 5, Wanda, a widow who occasionally teaches piano and organ in her home, purchased an electric organ from Murphys music store for $4,800, trading in her old organ for $1,200 and promising...
-
Choose the correct one: (a) \(S_{\mathrm{s}}>S_{\mathrm{l}}>S_{\mathrm{g}}\) (b) \(S_{\mathrm{s}} S_{\mathrm{s}}\) (d) \(S_{\mathrm{s}}>S_{1}
-
During the initial running-in period, usually the deterioration of a machine a. decreases b. increases c. remains constant
-
The financial statements at the end of Paradise Realty's first month of operations are shown below. PARADISE REALTY Statement of Cash Flows For the Month Ended November 30, 20Y3 Instructions 1. Would...
-
What is the competition in the construction industry, how can companies compete?
-
Maria sighed as she considered her new assignment. It had seemed like a great idea when Iris offered her the role, but now she wondered if she could get her arms around the complex process of getting...
-
a. In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. How many degrees of freedom are there for such a system? b. The reaction between nitrogen and...
-
Compute the partial molar volumes of methyl formate in methanolmethyl formate and ethanolmethyl formate mixtures at 298.15 K for various compositions using the experimental data in Fig. 8.1-2a and...
-
True or false: When an OSPF route sends its link state information, it is sent only to those nodes directly attached neighbors. Explain.
-
Preissle Company wants to sell some 20-year, annual interest, $1,000 par value bonds. Its stock sells for $45 per share, and each bond would have 75 warrants attached to it, each exercisable into one...
-
The common stock of Southern Airlines currently sells for $34, and its 10-years-to-maturity 9% convertible debentures (issued at par, or $1,000) sell for $850. Each debenture can be converted into 28...
-
Risky Holdings Company (RHC) has $48M in debt and a debt to total assets ratio of 40%. RHC would like to issue equity and use the proceeds to pay down its debt to reduce its debt to total assets...
-
6. NTS Inc. is investing in a new project that will generate expected cash flows of $10 starting in one year (t=1), which will grow at a rate of 5% through year t=7. After that, these cash flows are...
-
Dynamic Widgets, Inc. has received three different orders ( Job 1 0 1 , Job 1 0 2 , and Job 1 0 3 ) during the month of January. Each job is unique in terms of materials and labor required. The...
-
What two major pieces of legislation were adopted in the late 1980s and early 1990s to ameliorate the thrift crisis? Explain.
-
Write a function that reads a Float24_t value: Float24_t float24_read(void) A legitimate float24 value string is of the form: "mantissabexponent" where the mantissa (m) and the exponent (e) may have...
-
Channel-lock pliers hold a round metal bar as a machinist grips the handles with P = 50 N (Figure P4.21). Using the free body diagram shown for the combined lower jaw and upper handle, calculate the...
-
Refer to the exercise of Problem P4.21. (a) Measure the angle of force A directly from the diagram and use it to find the magnitude of the force at hinge B. (b) A design condition is that the force...
-
A pair of large hydraulically operated shears in Figure P4.23 is attached to the end of the boom on an excavator. The shear is used for cutting steel pipe and I-beams during demolition work....
-
Apex Limited (APL) is a management consultancy company headquartered in Singapore. After the successful implementation of a share option plan for the senior management team in 20x1, the company has...
-
7)A certain city covers an area of 62.294 mil 2 . How many square kilometers is this ?Use the conversion factor 1 mile = 1.61 km 8) A projectile is fired at 422.8 mi/hr. How fast is that in meters...
-
What is its unit price? Let's think of a batch of 100 baked goods...what is its total price? Speculate how much is the cost to bake (AND sell) that 1 batch (100 units)? Calculate its related gross...

Study smarter with the SolutionInn App


