The heat-of-mixing data of Featherstone and Dickinson [J. Chem. Thermodyn., 9, 75 (1977)] for the n-octanol +
Question:
The heat-of-mixing data of Featherstone and Dickinson [J. Chem. Thermodyn., 9, 75 (1977)] for the n-octanol + n-decane liquid mixture at atmospheric pressure is approximately fit by
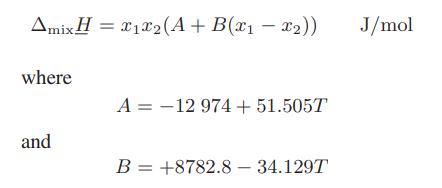
with T in K and x1 being the n-octanol mole fraction.
a. Compute the difference between the partial molar and pure-component enthalpies of n-octanol and of n-decane at x1 = 0.5 and T = 300 K.
b. Compute the difference between the partial molar and pure-component heat capacities of n-octanol and of n-decane at x1 = 0.5 and T = 300 K.
c. An x1 = 0.2 solution and an x1 = 0.9 solution are to flow continuously into an isothermal mixer in the mole ratio 2:1 at 300 K. Will heat have to be added or removed to keep the temperature of the solution leaving the mixer at 300 K? What will be the heat flow per mole of solution leaving the mixer?
Step by Step Answer:

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler





