From Eqs. 6.2-18 and 6.2-20, we have the following as definitions of the heat capacities at constant
Question:
From Eqs. 6.2-18 and 6.2-20, we have the following as definitions of the heat capacities at constant volume and constant pressure:
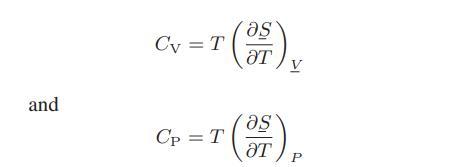
![as 'U C),-), ),-), [C).] = T V V V U V = Cv|T (6.2-18)](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/0/4/0/09265548d9c4aa281700040090435.jpg)
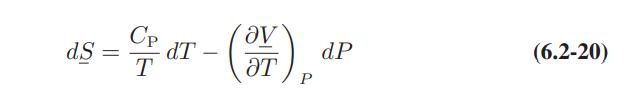
More generally, we can define a heat capacity subject to some other constraint X by
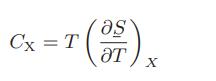
One such heat capacity of special interest is CLV, the heat capacity along the vapor-liquid equilibrium line.
a. Show that
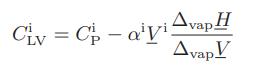
where αi is the coefficient of thermal expansion for phase i (liquid or vapor), CiP is its constantpressure heat capacity, Vi is its molar volume, and ΔvapH and ΔvapV are the molar enthalpy and volume changes on vaporization.
b. Show that
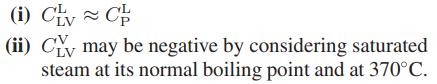
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





