Glucose and fructose both have the composition C 6 H 12 O 6 , but differ slightly
Question:
Glucose and fructose both have the composition C6H12O6, but differ slightly in structure, as shown below, and more so in sweetness. Repeat Illustration 15.7-9 using these sugars instead of xylose.
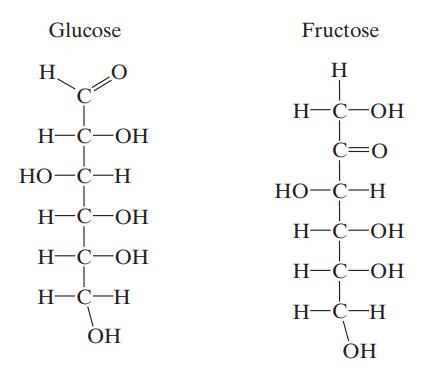
a. Estimate the maximum amount of 2,3-butanediol that can be produced per kg of glucose and fructose consumed, assuming no additional biomass production.
b. Estimate the amount of water and oxygen consumed, and carbon dioxide produced per C-mole of 2,3-butanediol produced assuming no additional biomass production.
c. Estimate the amount of heat released to keep the fermenter at 25°C per C-mole of 2,3-butanediol produced assuming no additional biomass production.
Illustration 15.7-9
Determining the Maximum Amount of Product That Can Be Obtained from a Substrate
A biologist is trying to genetically engineer bacteria to produce valuable organic chemicals from the inexpensive organic sugar xylose found in a variety of plants and berries. One such organism appears capable of producing 2,3-butanediol. The following data are available.
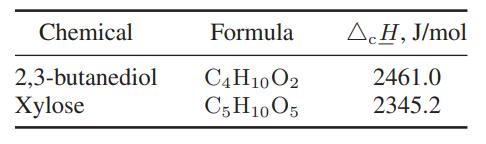
Step by Step Answer:

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler





