If the heat capacity of an ideal gas is given by Also develop expressions for this fluid
Question:
If the heat capacity of an ideal gas is given by
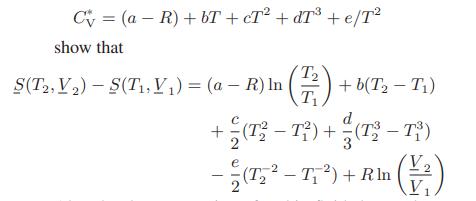
Also develop expressions for this fluid that replace Eqs. 4.4-3 and 4.4-4.
Transcribed Image Text:
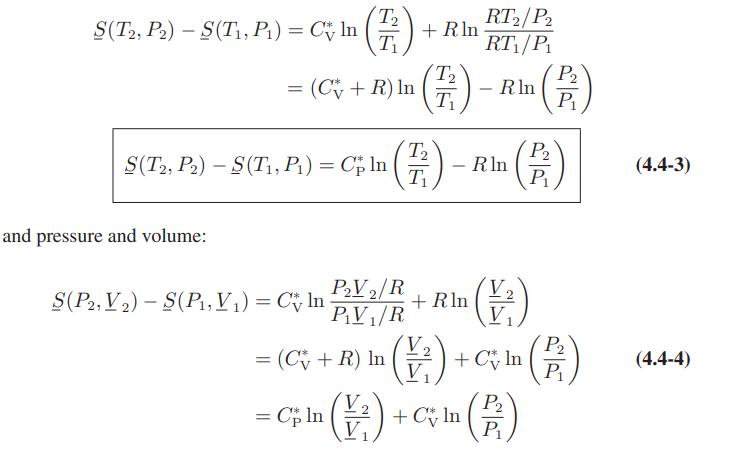
C = (a-R) +bT+cT²+dT³ + e/T² show that T₂ T₁ S(T2, V₂)-S(T₁, V₁) = (a - R) In + b(T₂ - T1) d +(T²-T²)+(13³-73) - (25²-7₁²) + Rlm (1²)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)

Answered By

Simon kingori
I am a tier-one market researcher and content developer who has been in this field for the last six years. I’ve run the freelancing gamut; from market research, data mining and SEO/SMM to copywriting, Content Development, you name it, I’ve done it. I’m extremely motivated, organized and disciplined – you have to be to work from home. My experience in Freelancing is invaluable- but what makes me a cut above the rest is my passion to deliver quality results to all my clients- it’s important to note, I've never had a dissatisfied client. Backed by a Masters degree in Computer Science from MOI university, I have the required skill set and burning passion and desire to deliver the best results for my clients. This is the reason why I am a cut above the rest. Having taken a Bsc. in computer science and statistics, I deal with all round fields in the IT category. It is a field i enjoy working in as it is dynamic and new things present themselves every day for research and exploration.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
Bob Burley and his brother Buford ran the best restaurant in Dallas, Texas. Many out-of-towners would visit Dallas and go to Burleys Biscuits, Beef, and Veggies for a good wholesome meal. One thing...
-
The specific heat at constant volume for an ideal gas is given by cv = 0.7 + (2.7 10 -4)T (kJ/kgK) where T is in kelvin. The change in the internal energy for this ideal gas undergoing a process in...
-
If the heat capacity of an ideal gas is C* P + 30 1 0.05T, with C* P in J/mol K and T representing the temperature in K, what is the change in internal energy and the change in enthalpy for five...
-
Research diversity expert Martin Davidson, author of The End of Diversity as We Know It: Why Diversity Efforts Fail and How Leveraging Difference Can Succeed. What is his direction for how you adopt...
-
Stroud and Freeman are general partners in Strouds Food Center, a grocery store. Nothing in the articles of partnership restricts the power or authority of either partner to act in respect to the...
-
Petty Cash The petty cash fund of Teasdale's Auto Repair Service, a sole proprietorship, contains the following. The general ledger account Petty Cash has a balance of $300. Prepare the journal entry...
-
Fantastic Sams and Defendants PSTEVO, LLC and Jeremy Baker entered into a franchise agreement pursuant to which Fantastic Sams granted PSTEVO a franchise to operate a Fantastic Sams Salon. According...
-
Aunt Mollys Old Fashioned Cookies bakes cookies for retail stores. The companys best-selling cookie is chocolate nut supreme, which is marketed as a gourmet cookie and regularly sells for $8.00 per...
-
In December of 2017, the US Government signed the Tax Cuts and Jobs Act (TCJA) into law. The TCJA had four goals; tax relief for middle-income families, simplification for individuals, economic...
-
The Ocean Thermal Energy Conversion (OTEC) project in Hawaii produces electricity from the temperature difference between water near the surface of the ocean (about 27 C) and the 600 m deep water at...
-
If it is necessary to compress hydrogen to a higher pressure than is possible with the single-compression step above, an alternative is to use two compressors (or a two-stage compressor) with...
-
The depreciation expense on office equipment for the month of March is $100. This is the third month that the office equipment, which cost $1,900, has been owned. Prepare the adjusting entry in...
-
Referring to the article "An Introduction to Embedded Systems and IoT" at http://www.inforcecomputing.com/blog/introduction-embedded-systems-iot/: What does IoT mean for an embedded developer?
-
Microsoft Windows is one of the most popular operating systems used in business. It is likely you will use Microsoft Windows in the healthcare industry, and your UMA computer uses Microsoft Windows....
-
Explain the concept of duality in the context of constrained optimization and its implications for understanding the interplay between objective functions and constraints in mathematical programming?
-
Consider the Fairfax Beach, located in Southwest portion of Freedonia. This beach is world renown for scenic views and sharkless waters. There is a boardwalk on this beach and this boardwalk is 3 km...
-
Suppose you are designing a "function" for a safety critical part of an automobile. This function can be implemented by one or more microprocessors and software. You can choose among three forms of...
-
List five asset accounts, three liability accounts, and five expense accounts included in the acquisition and payment cycle for a typical manufacturing company. Discuss.
-
What will be the final value of DI after executing the following piece of code? Execute the instructions dependently one after another. CLD MOU CX,OFOH MOU AX.02874H MOU DI,01000H MOU ES, DI SUB...
-
A shallow foundation measuring 1 m 2 m in plan is to be constructed over a normally consolidated sand layer. Given: D f = 1 m, N 60 increases with depth, N 60 (in the depth of stress influence) =...
-
A 2 m wide continuous foundation carrying a 260 kN/m wall load is placed at a depth of 1.0 m in sand where the unit weight is 19.0 kN/m 3 and (N 1 ) 60 is 32. Assuming Poissons ratio of 0.15,...
-
A 2 m 2 m foundation carrying a 1000 kN column load is placed at 1.0 m below the ground level in a sand where = 19 kN/m 3 and (N 1 ) 60 = 25. Estimate the settlement using the Berardi and...
-
la) What is the face chromatic number of the graph at the right? b) What is the edge chromatic number of the graph at the right? 2. Find the chromatic polynomial for the graph on the right. P A B L b...
-
You currently have a retirement portfolio balance of $800,000. You recently met with your financial advisor and determined you need to have $2.032 million saved before you can retire. If you are able...
-
A basis for S = span{[4 4 8 -16], [16 -16 32 -64], [1 1 0 0], [3 1 2 -4], [001 1]}. is B Check I

Study smarter with the SolutionInn App


