If it is necessary to compress hydrogen to a higher pressure than is possible with the single-compression
Question:
If it is necessary to compress hydrogen to a higher pressure than is possible with the single-compression step above, an alternative is to use two compressors (or a two-stage compressor) with intercooling. In such a process the hydrogen is compressed in the first stage of the compressor, then cooled at constant pressure to a lower temperature, and then compressed further in a second compressor or stage. Although it may not be economical to do so, more than two stages can be used.
a. Compute the maximum pressure that can be obtained in a two-stage compression with intercooling to 300 K between the stages, assuming hydrogen to be an ideal gas with the heat capacity given in Appendix A.II.
b. Repeat the calculation above for a three-stage compression with intercooling to 300 K.
Appendix A.II
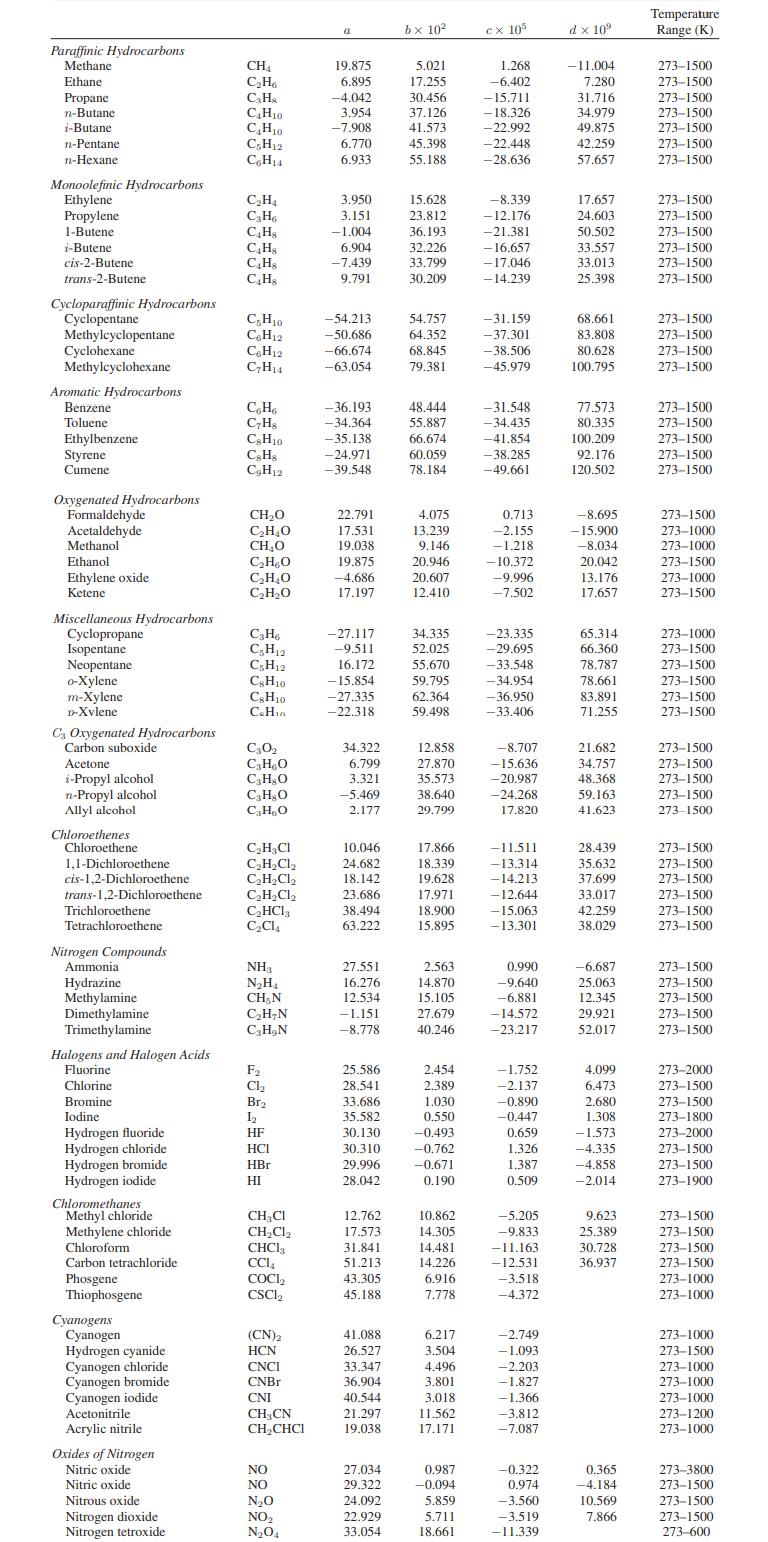
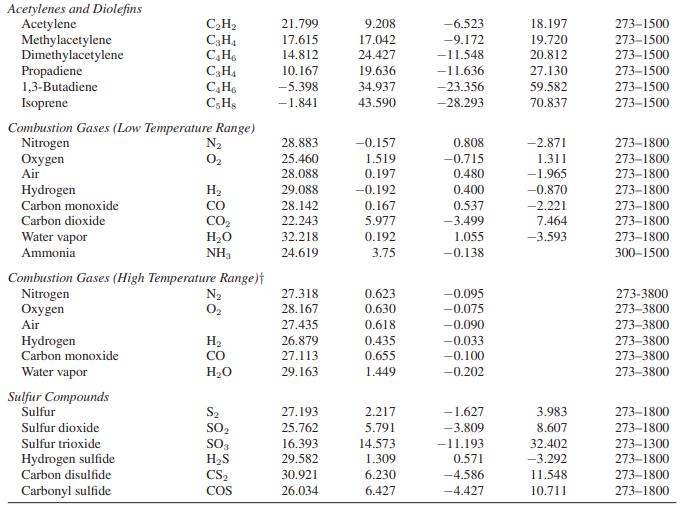
Paraffinic Hydrocarbons Methane Ethane Propane n-Butane i-Butane n-Pentane n-Hexane Monoolefinic Hydrocarbons Ethylene Propylene 1-Butene i-Butene cis-2-Butene trans-2-Butene Cycloparaffinic Hydrocarbons Cyclopentane Methylcyclopentane Cyclohexane Methylcyclohexane Aromatic Hydrocarbons Benzene Toluene Ethylbenzene Styrene Cumene Oxygenated Hydrocarbons Formaldehyde Acetaldehyde Methanol Ethanol Ethylene oxide Ketene Miscellaneous Hydrocarbons Cyclopropane Isopentane Neopentane o-Xylene m-Xylene D-Xvlene C3 Oxygenated Hydrocarbons Carbon suboxide Acetone i-Propyl alcohol n-Propyl alcohol Allyl alcohol Chloroethenes Chloroethene 1,1-Dichloroethene cis-1,2-Dichloroethene trans-1,2-Dichloroethene Trichloroethene Tetrachloroethene. Nitrogen Compounds Ammonial Hydrazine Methylamine Dimethylamine Trimethylamine Halogens and Halogen Acids Fluorine Chlorine Bromine Iodine Hydrogen fluoride Hydrogen chloride Hydrogen bromide Hydrogen iodide Chloromethanes Methyl chloride Methylene chloride Chloroform Carbon tetrachloride Phosgene Thiophosgene Cyanogens Cyanogen Hydrogen cyanide Cyanogen chloride Cyanogen bromide Cyanogen iodide Acetonitrile Acrylic nitrile Oxides of Nitrogen Nitric oxide Nitric oxide Nitrous oxide Nitrogen dioxide Nitrogen tetroxide CH₁ C₂H6 C₂H₂ C4H10 C₂H₁0 C5H12 C6H14 C₂H₁ C₂H6 C4H8 C4H8 C4Hs C4H8 C5H10 C6H12 C6H12 C₂H14 C6H6 C₂H8 CH10 CsH8 C₂H12 CH₂O C₂H₂O CH₂O C₂HBO C₂H₂O C₂H₂O C3H6 C₂H12 C5H12 CH10 C&H10 C.Hin C30₂ C3H₂O C3H8O C₂H₂O CHO C₂H₂Cl C₂H₂Cl₂ C₂H₂Cl₂ C₂H₂Cl₂ C₂HCl3 C₂C14 NH3 N₂H₁ CH, N C₂H₂N C₂H₂N F₂ Cl₂ Br₂ 1₂ HF HCI HBr HI CH₂Cl CH₂Cl₂ CHC13 CC14 COCI₂ CSCL (CN)2 HCN CNCI CNBr CNI CH3CN CH₂CHCI NO NO N₂O NO₂ N₂O4 a 19.875 6.895 -4.042 3.954 -7.908 6.770 6.933 3.950 3.151 -1.004 6.904 -7.439 9.791 -54.213 -50.686 -66.674 -63.054 -36.193 -34.364 -35.138 -24.971 -39.548 22.791 17.531 19.038 19.875 -4.686 17.197 -27.117 -9.511 16.172 -15.854 -27.335 -22.318 34.322 6.799 3.321 -5.469 2.177 10.046 24.682 18.142 23.686 38.494 63.222 27.551 16.276 12.534 -1.151 -8.778 25.586 28.541 33.686 35.582 30.130 30.310 29.996 28.042 12.762 17.573 31.841 51.213 43.305 45.188 41.088 26.527 33.347 36.904 40.544 21.297 19.038 27.034 29.322 24.092 22.929 33.054 bx 10² 5.021 17.255 30.456 37.126 41.573 45.398 55.188 15.628 23.812 36.193 32.226 33.799 30.209 54.757 64.352 68.845 79.381 48.444 55.887 66.674 60.059 78.184 4.075 13.239 9.146 20.946 20.607 12.410 34.335 52.025 55.670 59.795 62.364 59.498 12.858 27.870 35.573 38.640 29.799 17.866 18.339 19.628 17.971 18.900 15.895 2.563 14.870 15.105 27.679 40.246 2.454 2.389 1.030 0.550 -0.493 -0.762 -0.671 0.190 10.862 14.305 14.481 14.226 6.916 7.778 6.217 3.504 4.496 3.801 3,018 11.562 17.171 0.987 -0.094 5.859 5.711 18.661 cx 105 1.268 -6.402 -15.711 -18.326 -22.992 -22.448 -28.636 -8.339 -12.176 -21.381 -16.657 -17.046 -14.239 -31.159 -37.301 -38.506 -45.979 -31.548 -34.435 -41.854 -38.285 -49.661 0.713 -2.155 -1.218 -10.372 -9.996 -7.502 -23.335 -29.695 -33.548 -34.954 -36.950 -33.406 -8.707 -15.636 -20.987 -24.268 17.820 -11.511 -13.314 -14.213 -12.644 -15.063 -13.301 0.990 -9.640 -6.881 -14.572 -23.217 -1.752 -2.137 -0.890 -0.447 0.659 1.326 1.387 0.509 -5.205 -9.833 -11.163 -12.531 -3.518 -4.372 -2.749 -1.093 -2.203 -1.827 -1.366 -3.812 -7.087 -0.322 0.974 -3.560 -3.519 -11.339 d x 10⁹ -11.004 7.280 31.716 34.979 49.875 42.259 57.657 17.657 24.603 50.502 33.557 33.013 25.398 68.661 83.808 80.628 100.795 77.573 80.335 100.209 92.176 120.502 -8.695 -15.900 -8.034 20.042 13.176 17.657 65.314 66.360 78.787 78.661 83.891 71.255 21.682 34.757 48.368 59.163 41.623 28.439 35.632 37.699 33.017 42.259 38.029 -6.687 25.063 12.345 29.921 52.017 4.099 6.473 2.680 1.308 -1.573 -4.335 -4.858 -2.014 9.623 25.389 30.728 36.937 0.365 -4.184 10.569 7.866 Temperature Range (K) 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1000 273-1000 273-1500 273-1000 273-1500 273-1000 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-2000 273-1500 273-1500 273-1800 273-2000 273-1500 273-1500 273-1900 273-1500 273-1500 273-1500 273-1500 273-1000 273-1000 273-1000 273-1500 273-1000 273-1000 273-1000 273-1200 273-1000 273-3800 273-1500 273-1500 273-1500 273-600
Step by Step Answer:



Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Students also viewed these Engineering questions
-
Redo Problem 4.18 using Aspen Plus. Problem 4.18 If it is necessary to compress hydrogen to a higher pressure than is possible with the single-compression step above, an alternative is to use two...
-
A sample containing an alkali sulfate is dried, weighed and dissolved in dilute HCl. Barium chloride solution is added in excess to precipitate barium sulfate, and the precipitate is digested in the...
-
This is a research study, having four variables. .1)Leader humility 2)Antisocial behavior 3)mindfulness 4) Self-efficacy I need help to w.rite Abstract of this study and need you to make the sense...
-
There are several methods for this type of analysis. Vertical, horizontal,liquidity, profitability (which includes ratios), scenario and sensitivity, variance and valuation. As we review, do you feel...
-
Phillips and Harris are partners in a used car business. Under their oral partnership, each has an equal voice in the conduct and management of the business. Because of their irregular business...
-
What are the major elements in COBIT 2019?
-
Analyze the nature of ethnicity and its relationship to the nation-state.
-
Rylander Corporations condensed comparative income statements and balance sheets for 2014 and 2013 follow. Required 1. Prepare schedules showing the amount and percentage changes from 2013 to 2014...
-
EXPLAIN DIFFERENT WAYS IN WHICH YOU CAN PREPARE FOR SPECIFIED ROLES related to HRM?
-
If the heat capacity of an ideal gas is given by Also develop expressions for this fluid that replace Eqs. 4.4-3 and 4.4-4. C = (a-R) +bT+cT+dT + e/T show that T T S(T2, V)-S(T, V) = (a - R) In + b(T...
-
One mole of carbon dioxide is to be compressed adiabatically from 1 bar and 25 C to 10 bar. Because of irreversibilities and poor design of the compressor, the compressor work required is found to...
-
Integrate z 2 /(z 2 - 1) by Cauchys formula counterclockwise around the circle. |z - 1 - i| = /2
-
Develop at least two research questions and two hypotheses directly related to Veteran Homelessness and Mental illness. When constructing your questions and hypotheses, consider the guidelines stated...
-
Using the following information, determine the balance of the cash account. The left side of the T account sums to $34,500, and the right side of the T account sums to $19,450. a. $15,050 b. $53,950...
-
Discuss the evolutionary dynamics and molecular mechanisms driving speciation and diversification in eukaryotic organisms, exploring concepts such as allopatric, sympatric, and parapatric speciation,...
-
Dear Students, you have viewed the PowerPoint on Mr. Akihiko Kondo and his marriage in 2018 to Miku -a Japanese Anime character; and engaged with link included in module for Gatebox - the Japanese...
-
Adams Pet Supplies purchases its inventory from a variety of suppliers, some of which require a six-week lead time before delivering the goods. To ensure that she has a sufficient supply of goods on...
-
What are points on a mortgage? What factors does a mortgage borrower need to consider when deciding whether or not to take points on a mortgage?
-
Find the area of the surface generated by revolving the para- metric curve x = cos 1, y = sin? 1 (0 < I sa/2) about the y-axis.
-
A 3.0 m wide square foundation is placed at 1.5 m depth in sand where = 18.5 kN/m 3 . The water table lies well below the foundation level. Under the applied pressure of 200 kN/m 2 at the foundation...
-
Refer to the rectangular combined footing in Figure 10.1, with Q 1 = 500 kN and Q 2 = 750 kN. The distance between the two column loads, L 3 = 4.5 m. The proximity of the property line at the left...
-
Refer to the trapezoidal combined footing in Figure 10.2, with Q 1 = 1000 kN and Q 2 = 500 kN. The distance between the columns L3 is 4 m, and the net allowable soil pressure is 125 kN/m 2 . It is...
-
Based in Miramichi, New Brunswick, Abenaki Associates Ltd. has been providing information and computer software technology to First Nation customers for more than thirty years. Abenaki Associates is...
-
On March 1, 2025, Blossom Construction Company contracted to construct a factory building for Bridgeport Manufacturing Inc. for a total contract price of $8,430,000. The building was completed by...
-
As you lead your organization's performance management efforts, your chief human resources officer has real concerns about the buy-in among your staff. She has come to you to share her thoughts. With...

Study smarter with the SolutionInn App


