In a continuous manufacturing process, chlorodifluoromethane (CHClF 2 ), initially at 10 bar and 420 K, passes
Question:
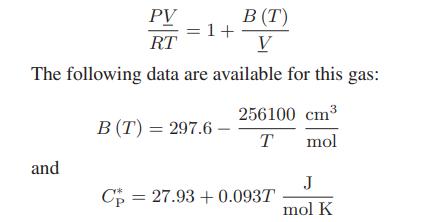
In a continuous manufacturing process, chlorodifluoromethane (CHClF2), initially at 10 bar and 420 K, passes through an adiabatic pressure-reducing valve so that its pressure drops to 0.1 bar (this last pressure low enough that CHClF2 can be considered to be an ideal gas). At these operating conditions, the gas can be represented by a one-term virial equation of state:
What is the temperature of the chlorodifluoromethane exiting the valve?
Transcribed Image Text:
PV RT The following data are available for this gas: and = 1+ B (T) V B (T) = 297.6 - 256100 cm³ T mol Cp 27.93 +0.093T = J mol K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
Approach Since the pressurereducing valve is adiabatic there is no heat transfer into or out of the ...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
In a continuous manufacturing process, chlorodifluoromethane (CHClF 2 ) initially at 10 bar and 420 K, passes through an adiabatic pressure reducing valve so that its pressure is 0.1 bar (this last...
-
In a continuous manusfacturing process chlorodifluoromethane (CHClF 2 ) initially at 10 bar and 420C passes through and adiabatic pressure reducing valve so that its pressure is reduced to 0.1 bar...
-
How much entropy is generated per mole of chlorodifluoromethane that passes through the pressurereducing valve of the previous problem? Data From Previous Probem In a continuous manufacturing...
-
Transform the following product by making the change of variable i = k + 1. k So when k and K +2 When k = 1, then i = n k II k +2 k = 1 = n+ 1 II i = 2 are expressed in terms of i, the results are k...
-
Jones, having filed locally an affidavit required under the assumed name statute, has been operating and advertising his exclusive toy store for twenty years in Centerville, Illinois. His advertising...
-
Phosphorus pentachloride dissociates according to the reaction: \[ \mathrm{PCl}_{5}(\mathrm{~g}) ightarrow \mathrm{PCl}_{3}(\mathrm{~g})+\mathrm{Cl}_{2}(\mathrm{~g}) \] Show that...
-
Analyze the relationship between women's rights, culture, and national prosperity.
-
Regression, activity-based costing, choosing cost drivers Fitzgerald Manufacturing has been using activity-based costing to determine the cost of product X-678. One of the activities, "Inspection,"...
-
what is the present value of $4000 per year at a discount rate of 10 percent if the interest if the first payment is received in 8 years from now and the last in 25 years from now
-
A gas is continuously passed through an adiabatic turbine at the rate of 2 mol/s. Its initial temperature is 600 K, its initial pressure is 5 bar and its exiting pressure is 1 bar. Determine the...
-
The Boyle temperature is defined as the temperature at which the second virial coefficient B is equal to zero. a. Recognizing that any equation of state can be expanded in virial form, find the Boyle...
-
For each of the functions in Exercises 11 through 14, compute the second-order partial derivatives f xx , f yy , f xy , and f yx . f(x, y) = e x2+y 2
-
Plaintiff, Joseph H. Brotman, filed a bill of complaint against his wife, Miriam, to recover possession of certain machinery, equipment and material. Later, he brought a second suit in equity against...
-
1. Discuss the three (3) legal mechanisms involved in the passing of title. 2. Distinguish between a contract to sell and a contract for sale. 3. How is an agency created? 4. Describe the three (3)...
-
Review the local print media (newspaper/magazine/web) and identify a Canadian crime story that is of interest to you.The story does not require that the police investigation has come to a conclusion...
-
On January 30, a company that designs and builds generators to standard industrial specifications received a telephone call from a buyer who ordered two generators at a price of $25,000 each. The...
-
Chris grew up in New York. After attending college in New York, he decided to go to law school in Florida. When he started law school he signed up for the Florida bar exam, because he decided that he...
-
If a bundle of goods in Japan costs 4,000,000 while the same goods and services cost $ 40,000 in the United States, what is the current exchange rate of U.S. dollars for yen? If, over the next year,...
-
Interest Compounded Annually. When P dollars is invested at interest rate i, compounded annually, for t years, the investment grows to A dollars, where A = P(1 + i) t . Trevor's parents deposit $7800...
-
Consider the following equation. a. Plot the free response for the initial conditions y(0) = 10,y(0) = -5. b. Plot the unit-step response (for zero initial conditions). c. The total response to a...
-
Draw a simulation diagram for the following equation. = 5f(t) 7y
-
The model for the RC circuit shown in Figure P42 is For RC = 0.2 s, plot the voltage response o (t) for the case where the applied voltage is a single square pulse of height 10 V and duration 0.4 s,...
-
10.) How many iterations will the following loop execute? int intIndex = 100; while (int Index < 10) Console.WriteLine ("hello"); int Index += 1;
-
5. [10 points] Consider the following graph: 2 5 3 6 a) Starting at vertex 1 and resolving ties by vertex d number, traverse the graph by depth- first search and construct the corresponding...
-
How many processes are created by the following program, including the initial process. (.5 Marks) #include #include int main() { int i; for (i=0; i <5; i++) fork(); return 0; ANSWER:

Study smarter with the SolutionInn App


