In this chapter, from the third law of thermodynamics, it has been shown that the entropy of
Question:
In this chapter, from the third law of thermodynamics, it has been shown that the entropy of all substances approaches a common value at 0 K (which for convenience we have taken to be zero). This implies that the value of the entropy at 0 K is not a function of volume or pressure. Use this information to show the following:
CV = 0
at T = 0K and that the coefficient of thermal expansion
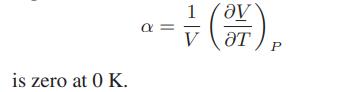
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





