Methane gas hydrates are formed from liquid water by the following reaction: a. Calculate the Gibbs energy
Question:
Methane gas hydrates are formed from liquid water by the following reaction:
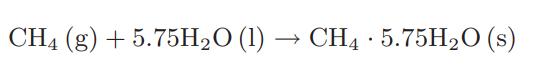
a. Calculate the Gibbs energy of formation of the hydrate at 278 K and 283 K using the information that the methane partial pressure in equilibrium with the hydrate at 278 K is 4.2 MPa and at 283 K is 6.8 MPa.
b. One common way to prevent hydrates from forming is by adding an inhibitor to the system, usually methanol or a salt (e.g., NaCl). If 10 wt % methanol is added to water, what will be the equilibrium partial pressure for methane for hydrate formation at 278 K and 283 K (assume no methanol is present in the vapor)?
c. What phases and components are present at equilibrium at 273.15 K? How many degrees of freedom are there for this system?
Step by Step Answer:

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler





