Redo Problem 13.1 using Aspen Plus. Problem 13.1 Isopropyl alcohol is to be dehydrogenated in the gas
Question:
Redo Problem 13.1 using Aspen Plus.
Problem 13.1
Isopropyl alcohol is to be dehydrogenated in the gas phase to form propionaldehyde according to the reaction
Compute the equilibrium fraction of isopropyl alcohol that would be dehydrogenated at 500 K and 1.013 bar.
Transcribed Image Text:
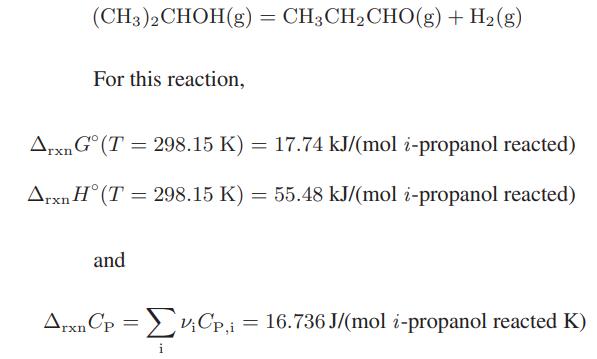
(CH3)2CHOH(g) = CH3CH₂CHO(g) + H₂(g) For this reaction, Arxn Gᵒ (T= 298.15 K) = 17.74 kJ/(mol i-propanol reacted) Arxn H (T = 298.15 K) = 55.48 kJ/(mol i-propanol reacted) and ArxnCp = V₁Cp,i = 16.736 J/(mol i-propanol reacted K) ΣvCp, i
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)

Answered By

Nandana Wijayarathna
I am a highly experienced writer in several areas,
Business management
Information technology
Business administration
Literature
Biology
Environmental science
History
4.50+
161+ Reviews
399+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
Isopropyl alcohol is to be dehydrogenated in the gas phase to form propionaldehyde according to the reaction Compute the equilibrium fraction of isopropyl alcohol that would be dehydrogenated at 500...
-
An electroplating process supplies a coating thickness of 800 nm. Calculate the area which would be covered using 1 kg of gold, mindful that the density of gold is 19300 kg/m3
-
The decomposition of iodoethane in the gas phase proceeds according to the following equation: C2H5I(g) C2H4(g) + HI(g) At 660. K, k = 7.2 10-4 s-1; at 720. K, k = 1.7 10-2 s-1. What is the rate...
-
A jet is traveling westward with the sun directly overhead (the jet is on a line between the sun and the center of the Earth). How fast must the jet fly in order to keep the sun directly overhead?...
-
An effective budget converts the goals and objectives of an organization into data. The budget serves as a blueprint for managements plans. The budget is also the basis for control. Management...
-
The following is Ash's trial balance as at 31 March 2011: Additional information: 1 Stock at 31 March 2011: 15 000. 2 At 31 March 2011 there was a specific bad debt of 6000. This was to be written...
-
Do larger butterflies live longer? The wingspan (in millimeters) and the lifespan in the adult state (in days) were measured for 22 species of butterfly. Following are the results. a. Compute the...
-
The following information pertains to peak heights company: Required: Present the operation activities section of the statement of cash flows for peak heights company using the indirectmethod. Income...
-
AHP, Inc. (AHP) is expected to generate $35.0 million in revenue next year, $22.0 million in EBITDA (earnings before interest, taxes, depreciation and amortization) and $15.0 million in EBIT...
-
Adenosine monophosphate (AMPH) is a nucleotide that is present as a monomer in DNA and RNA. It consists of a phosphate group, a ribose molecule and an adenine molecule. Consequently, it can be found...
-
Redo Problem 13.3 using Aspen Plus. Problem 13.3 Carbon dioxide can react with graphite to form carbon monoxide, and the carbon monoxide formed can further react to form carbon and oxygen: Determine...
-
Use the transforms in Fig. 10.1.2 to find the Laplace transforms of the functions in Problems 11 through 22. A preliminary integration by parts may be necessary. f (t) = t 3/2 - e -10t t f(t) t" (n...
-
Where did they get 8550 for patient days?? patients x days in month 95 x 30=2,850 not 8550. Monthly Food Cost Number of Patients Days in the month Patient Days PPD $16,356.00 95 30
-
1) Write a class called Room, which has three private instance variables: a) a double width, representing the width of the room in feet, b) a double length, representing the length of the room in...
-
Congratulations, on being appointed as the director of the North Carolina Open Singles Badminton Championships. You have been researching probable revenues and costs so that you can make a budget....
-
Discuss the following forms of share buy-backs permitted in Australia. Include a short description of the characteristics of each form and any legal conditions that are imposed: Equal access...
-
= The electric field component of an electromagnetic plane wave traveling in a vacuum is given by = Eo sin (kx+ wt), where E=300 V/m and k=107%m. What is the magnetic field component of the...
-
Beta Corporation has the following shareholders equity accounts Common stock at par ........... $ 5,000,000 Paid-in capital in excess of par ....... 2,000,000 Retained earnings ...............
-
Time Solutions, Inc. is an employment services firm that places both temporary and permanent workers with a variety of clients. Temporary placements account for 70% of Time Solutions' revenue;...
-
Some American companies have refused to promote women into positions of high authority in their international operations in Asia, the Middle East, and South America. Their rationale is that business...
-
Marvin Johnson is an Environmental Engineer for one of several local plants whose water discharges flow into a lake in a flourishing tourist area. Included in Marvins responsibilities is the...
-
Derek Evans used to work for a small computer firm that specializes in developing software for management tasks. Derek was a primary contributor in designing an innovative software system for...
-
Benzene (C6H6) is converted to cyclohexane (C6H12) by direct reaction with H2. The fresh feed to the process is 260 L/min of C6H6 plus 950 L/min of H2 at 100 degrees Celsius and 150 kPa. The single...
-
Simplify: 4(9x-8) -2(x-9)
-
Refer to video https://www.youtube.com/watch?v=b2phdK9lmsQ 1) What is Paul trying to convey to his audience about tangible and intangible thoughts? 2) What is the difference in how the market values...

Study smarter with the SolutionInn App


