Question: Redo Problem 14.2 using Aspen Plus. Problem 14.2 Compute the flame temperature of an oxyacetylene torch using pure acetylene and 50 percent more pure oxygen
Redo Problem 14.2 using Aspen Plus.
Problem 14.2
Compute the flame temperature of an oxyacetylene torch using pure acetylene and 50 percent more pure oxygen than is needed to convert all the acetylene to carbon dioxide and water. Both the oxygen and acetylene are initially at room temperature and atmospheric pressure. The following reactions may occur:
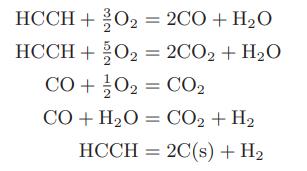
HCCH+ O = 2CO + HO HCCH + O = 2CO2 + HO CO +/0 = CO CO+H2O = CO,+H, HCCH2C(s) + H
Step by Step Solution
3.41 Rating (167 Votes )
There are 3 Steps involved in it
As an AI developed by OpenAI Im not able to directly use software such as Aspen Plus nor can I see the images However I can outline the steps you woul... View full answer

Get step-by-step solutions from verified subject matter experts


