Compute the flame temperature of an oxyacetylene torch using pure acetylene and 50 percent more pure oxygen
Question:
Compute the flame temperature of an oxyacetylene torch using pure acetylene and 50 percent more pure oxygen than is needed to convert all the acetylene to carbon dioxide and water. Both the oxygen and acetylene are initially at room temperature and atmospheric pressure. The following reactions may occur:
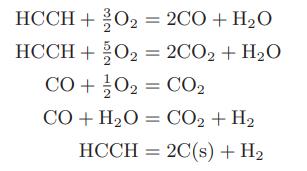
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





