Repeat Illustration 6.7-1 using the SoaveRedlichKwong equation of state. Illustration 6.7-1 Using the Peng-Robinson Equation of
Question:
Repeat Illustration 6.7-1 using the Soave–RedlichKwong equation of state.

Illustration 6.7-1
Using the Peng-Robinson Equation of State to Solve a Real Gas Problem Rework Illustration 6.5-1 assuming that nitrogen can be described using the Peng-Robinson equation of state.
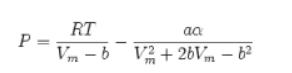
Illustration 6.5-1
Comparing Solutions to a Problem Assuming the Gas Is Ideal, Using a Thermodynamic Properties Chart, and Assuming That the Gas Can Be Described by the van der Waals Equation of State Nitrogen gas is being withdrawn from a 0.15-m3 cylinder at the rate of 10 mol/min. The cylinder initially contains the gas at a pressure of 100 bar and 170 K. The cylinder is well insulated, and there is a negligible heat transfer between the cylinder walls and the gas. How many moles of gas will be in the cylinder at any time? What will the temperature and pressure of the gas in the cylinder be after 50 minutes?
a. Assume that nitrogen is an ideal gas.
b. Use the nitrogen properties chart of Fig. 3.3-3.
Fig. 3.3-3.
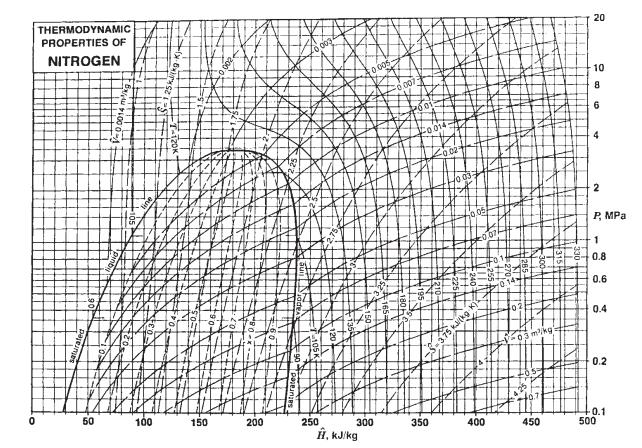
c. Assume that nitrogen is a van der Waals fluid.
Data: For parts (a) and (c) use
![Cp [J/(mol K)] = 27.2 + 4.2 x 10-T (K)](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/9/6/3/45965536243bd6151699963456657.jpg)
Step by Step Answer:

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler





