Starting with an equimolar mixture of lactate and NAD+ at 25C, calculate and plot the extent of
Question:
Starting with an equimolar mixture of lactate and NAD+ at 25°C, calculate and plot the extent of the following reaction as a function of pH:
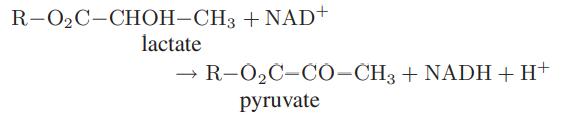
where R is a side group. For this reaction at solution conditions, the apparent Gibbs energy change is ΔrxnG = 25.9 kJ. Also determine the pH at which half the lactate will have reacted at equilibrium.
Transcribed Image Text:
R-O₂C-CHOH-CH3 + NAD+ lactate R-O₂C-CO-CH3 + NADH + H+ pyruvate
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
Calculating the extent of the reaction as a function of pH We can use the HendersonHasselbalch equation to calculate the fraction of lactate that is d...View the full answer

Answered By

Talha Talib
I am a member of IEEE society. As i am a student of electrical engineering badge 17 but beside of this i am also a tutor in unique academy. I teach calculus, communication skills, mechanics and economics. I am also a home tutor. My student Muhammad Salman Alvi is a brilliant A-level student and he performs very well in academics when i start to teach him. His weak point was mathematics but now he is performing well in mathematics. I am a scholarship holder in Fsc as i scored 1017 marks in metric out of 1100. Later on i got scholarship in Punjab Group of Colleges. I got 2nd position in robotics competition in 2018 as my project home automation select for the exhibition in Expocentre.
4.60+
23+ Reviews
62+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
Starting with an equimolar mixture of acetaldehyde and NAD + at 25C, calculate and plot the extent of the following reaction as a function of pH. Also determine the pH at which half the acetaldehyde...
-
Starting with an equimolar mixture of methanol and NAD + at 25C, calculate and plot the extent of the following reaction as a function of pH: For this reaction at solution conditions, the apparent...
-
An equimolar mixture of oxygen and nitrogen enters a compressor operating at steady state at 10 bar, 220 K with a mass flow rate (m) of 1 kg/s. The mixture exits the compressor at 60 bar, 400 K with...
-
Solve the right triangles with the given parts or state that there is not enough information to solve. Round off results according to Table 4.1. Refer to Fig. 4.37. B = 32.1, c = 238 Data from Table...
-
Assume that a company has decided not to allocate any support service costs to producing departments. Describe the likely behavior of the managers of the producing departments. Would this be good or...
-
Delaware Bay Chemical Company produces three products: ethylene, butane, and ester. Each of these products has high demand in the market, and Delaware Bay Chemical is able to sell as much as it can...
-
When conducting an incremental analysis, what step must always be taken immediately prior to beginning the pairwise comparisons? a. Order the alternatives from highest to lowest initial investment b....
-
Glenn Foreman, president of Oceanview Development Corporation, is considering submitting a bid to purchase property that will be sold by sealed bid at a county tax foreclosure. Glenns initial...
-
Prove that if M is a dense linear subspace of a separable Hilbert space H, then H has an orthonormal basis consisting of elements in M. Does the same result hold for arbitrary dense subsets of H?
-
Determine the charge on tyrosine as a function of pH. Tyrosine (C 9 H 11 NO 3 ), another amino acid in proteins, has two dominant ionizable groups with pK HA values of 2.24 and 9.04 at 25C (and a...
-
Adenosine monophosphate (AMPH) is a nucleotide that is present as a monomer in DNA and RNA. It consists of a phosphate group, a ribose molecule and an adenine molecule. Consequently, it can be found...
-
The number of pounds of steam used per month by a chemical plant is thought to be related to the average ambient temperature (inoF) for that month. The past years usage and temperature are shown in...
-
Many publicly traded financially distressed firms are purchased by private equity funds and delisted from the stock exchange. Several years later they are brought back to the exchange for a new share...
-
Why would a corporation wish to combine put and call options on a commodity? Provide an example of a case where this might happen.
-
What are the advantages and disadvantages of issuing warrants?
-
The Pirelli & C. SpA share price is 8.895. A call option with an exercise price of 9 sells for 0.35 and a put option with the same exercise price sells for 0.65. Does this make sense? Explain.
-
In 2012, Rangers Football Club went into administration with over 100 million in debt. The owner of the firm, Craig Whyte, held 85.3 per cent of the clubs shares and was also its only secured...
-
Cameron Bly is a sales manager for an automobile dealership. He earns a bonus each year based on revenue from the number of autos sold in the year less related warranty expenses. Actual warranty...
-
Consider the advantages and disadvantages of extending property rights so that everyone would have the right to prevent people imposing any costs on them whatsoever (or charging them to do so).
-
Locate the centroid x of the area. y h
-
Draw a conceptual sketch of your computer. Identify the keyboard, screen, power source, and information storage devices using arrows and labels.
-
Repeat Example 1.3 using the NSPE Code of Engineering Ethics. Solve using the Engineering Ethics Matrix. Example 1.3 The following scenario is a common situation faced by engineering students. Read...
-
You have observed the following returns over time Year 2014 2015 2016 2017 2018 Stock A Stock B Market 10% 13% 12% 15% 13% 10% 12% 13% 12% -1% 13% 1% 16% 13% 15% What are the betas of Stocks A and B?
-
Is the United States governable as a large republic? in the form of an op-ed, similar to those that appear in major newspapers. Your op-ed must address the following: What position did the Federalist...
-
Consider a project with the following cash flows: Year 0 Cash Flow - 12000 1 234 4000 4000 4000 4000 If the appropriate discount rate for this project is 15%, then the net present value (NPV) is

Study smarter with the SolutionInn App


