Starting with an equimolar mixture of methanol and NAD + at 25C, calculate and plot the extent
Question:
Starting with an equimolar mixture of methanol and NAD+ at 25°C, calculate and plot the extent of the following reaction as a function of pH:
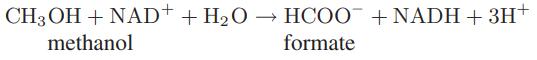
For this reaction at solution conditions, the apparent Gibbs energy change is ΔrxnG = −15.1 kJ. Also, assume that water is present in great excess, so that its concentration does not change in the course of the reaction.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





