The data below are for the activity coefficients of lithium bromide in aqueous solutions as a function
Question:
The data below are for the activity coefficients of lithium bromide in aqueous solutions as a function of molality at 25°C
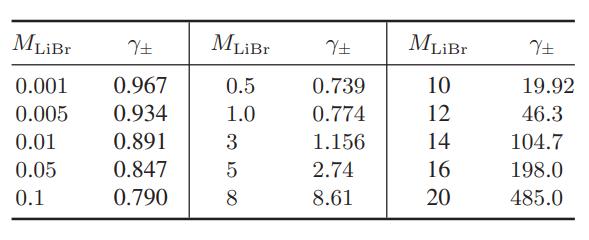
Compare the predictions of the Debye-Huckel model (Eqs. 9.10-15 and 9.10-16), and the extended DebyeHuckel models (Eqs. 9.10-17 and 9.10-18) with these data.


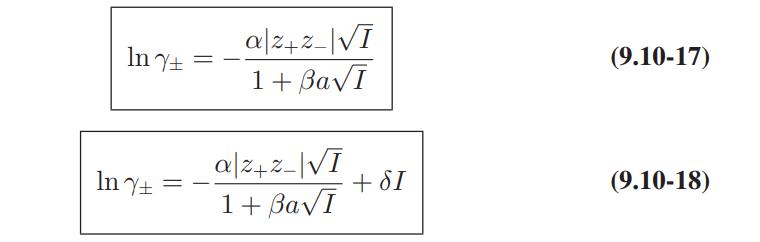
Transcribed Image Text:
MLiBr Y+ 0.001 0.967 0.005 0.934 0.01 0.891 0.05 0.847 0.1 0.790 MLiBr 0.5 1.0 Y+ 0.739 0.774 3 1.156 5 2.74 8 8.61 MLiBr 10 12 14 16 20 Y+ 19.92 46.3 104.7 198.0 485.0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
The following data are available for mean activity coefficients of single electrolytes in water at 25C. Compare these data with the predictions of the DebyeHuckel limiting law, Eq. 9.10-15, and Eq....
-
Figure 11-31 shows a cooling curve for a Pb-Sn alloy. Determine (a) The pouring temperature; (b) The superheat; (c) The liquidus temperature; (d) The eutectic temperature; (e) The freezing range; (f)...
-
Refer to the Nike information in Exhibit 4-5. Using the data for fiscal year 2001, compute both the operating income and the operating income as a percentage of sales. Treat the restructuring charge...
-
What are norms and values? Do people within a culture agree upon them?
-
What is the principle of mode superposition? What is its use in modal analysis?
-
On January 1, Paisley, Inc., paid $560,000 for all of Skyler Corporations outstanding stock. This cash payment was based on a price of $180 per share for Skylers $100 par value preferred stock and...
-
Given the following free cash flows. determine the IRR for the three independent projects A, B, and C. Data Table Initial outlay (Click on the following icon in order to copy its contents into a...
-
X, Y and Z are in partnership sharing profits and losses in the ratio 4 : 2 : 2. Z died on 30 June 20X2. The partnership statement of financial position as at that date was: Additional information It...
-
Experimentally it is observed that This equation implies that the activity coefficient i (or its logarithm) is weakly dependent on mole fraction near the pure component limit. Since we also know...
-
There are several possible expressions that can be used for the Gibbs excess energy. One is the Redlich-Kister expansion where B = 0, but A and C are nonzero. Find expressions for the activity...
-
Assume that the standard deviation of monthly rents paid by students in a particular town is $40. A random sample of 100 students was taken to estimate the mean monthly rent paid by the whole student...
-
Calculate the change in accounts receivable based on the transactions below that took place in the same period: Cash sales: $650 Sales on credit: $780 Receipt from receivable: $420
-
The following data is from Chance Co.'s accounting records for year 2019: Units produced and sold Total revenues and costs Sales revenue Direct materials costs Direct labor costs Variable...
-
Anderson Corp. has the following information: Beginning Ending Inventory Inventory (1/1) (12/31) Direct Materials Inventory $20,000 $30,000 Work in Process Inventory $16,000 $18,000 Finished Goods...
-
The following is the adjusted trial balance of Sierra Company. Sierra Company Adjusted Trial Balance Account Title Cash December 31 Prepaid insurance Notes receivable (due in 5 years) Buildings...
-
A 2kg mass is moving at 10m/s along a frictionless surface towards a spring (at rest) with spring constant 50N/m. When the mass hits the spring, it will begin to compress it. What is the maximum...
-
How does the degree of liquidity risk differ for different types of financial institutions?
-
Show that the block upper triangular matrix A in Example 5 is invertible if and only if both A 11 and A 22 are invertible. Data from in Example 5 EXAMPLE 5 A matrix of the form A = [ A11 A12 0 A22 is...
-
(a) If a diffraction grating has a resolution of 10 4 , is it possible to distinguish two spectral lines with wavelengths of 10.00 and 10.01 m? (b) With a resolution of 10 4 , how close in...
-
The true absorbance of a sample is 1.000, but the monochromator passes 1.0% stray light. Add the light coming through the sample to the stray light to find the apparent transmittance of the sample....
-
Refer to the Fourier transform infrared spectrum in Figure 19-32. (a) The interferogram was sampled at retardation intervals of 1.2660 10 -4 cm. What is the theoretical wavenumber range (0 to ?) of...
-
Discuss the role of process simulation in the design and evaluation of sustainable processes. How can Life Cycle Assessment (LCA) and simulation be combined to optimize environmental impacts and...
-
Explain the significance of multi-scale modeling in process simulation. How does the integration of different scales, from molecular to plant-level simulations, provide a comprehensive view of...
-
Answer the following questions regarding a new California state Law, https://leginfo.legislature.ca.gov/faces/billNavClient.xhtml?bill_id=202120220AB89...

Study smarter with the SolutionInn App


