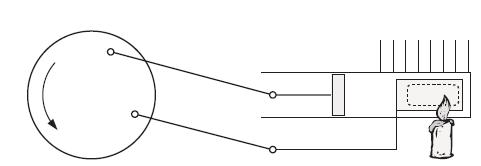
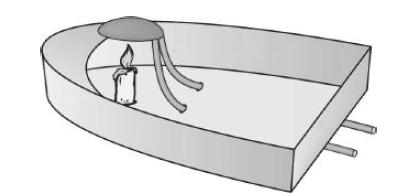
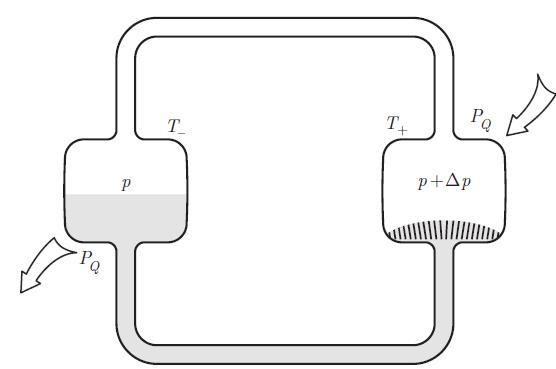
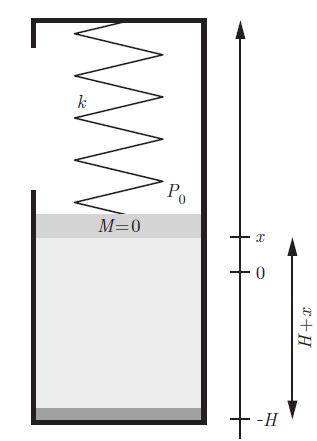
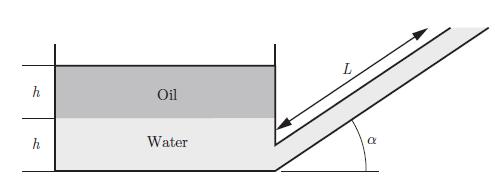
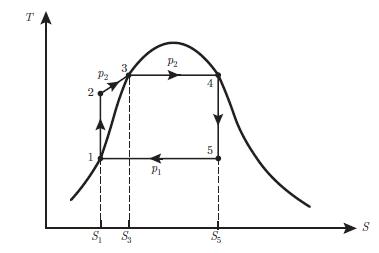
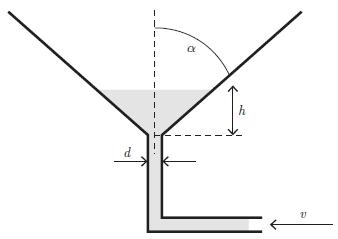
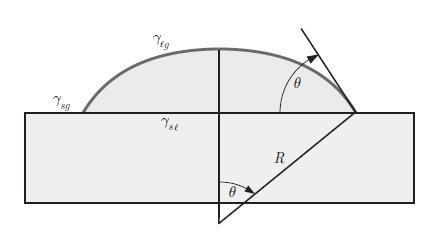
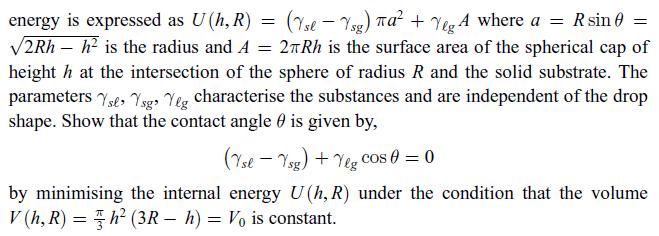
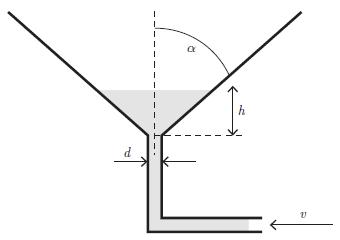
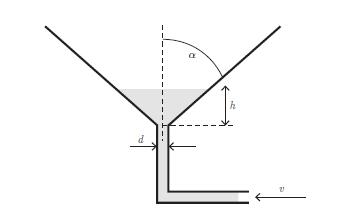
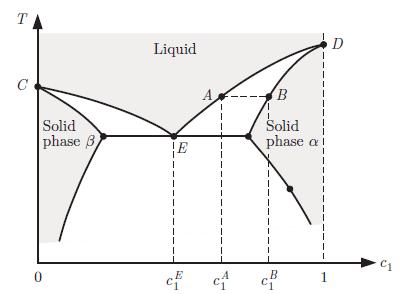
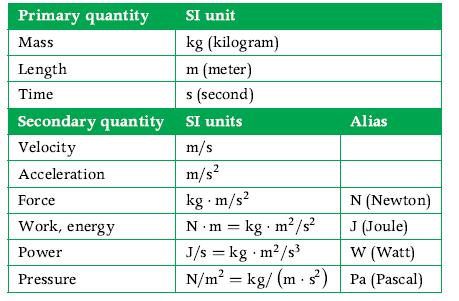
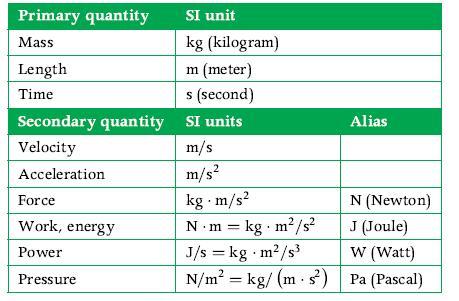
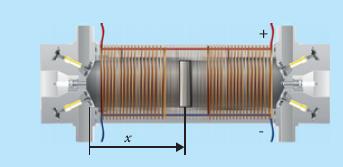
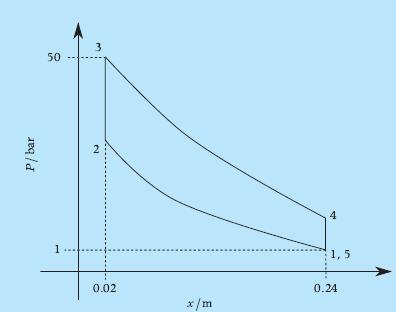
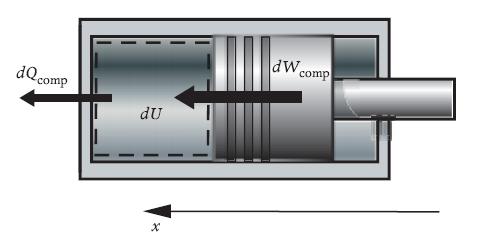
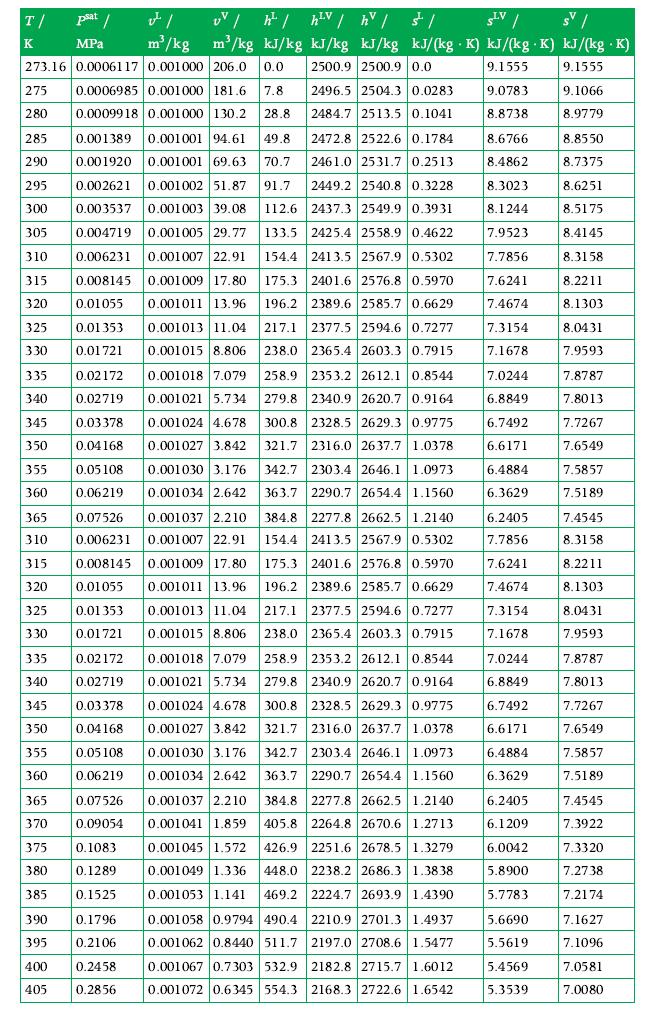
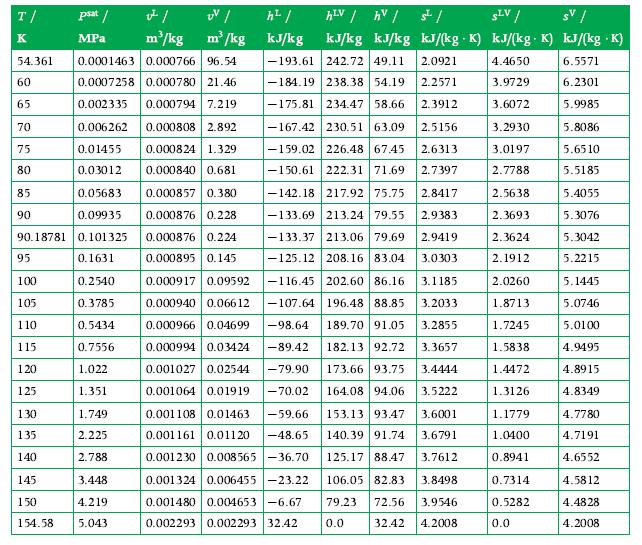
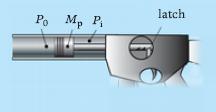
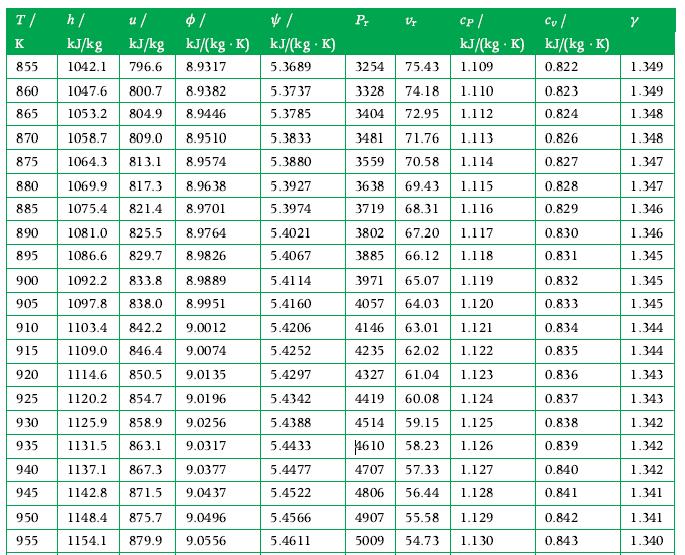
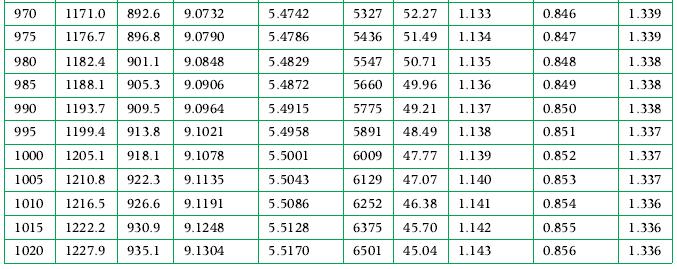
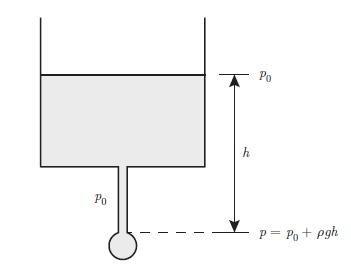
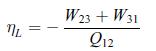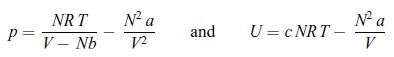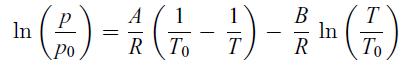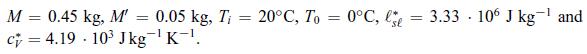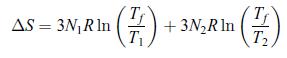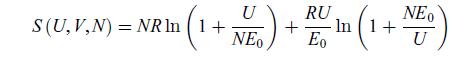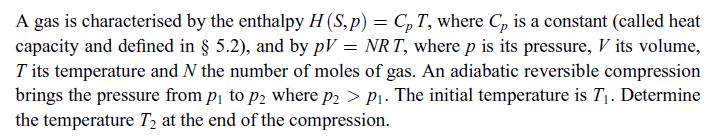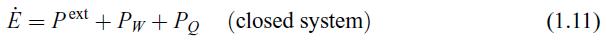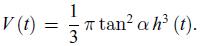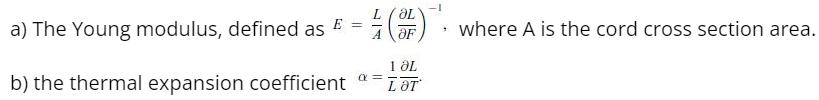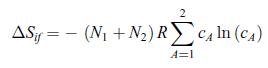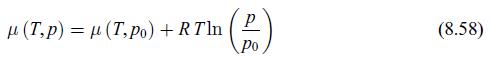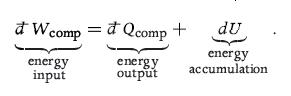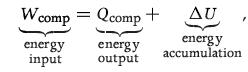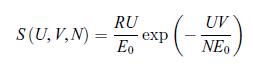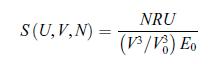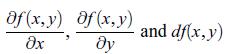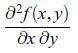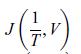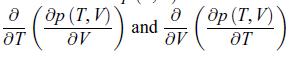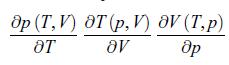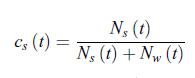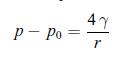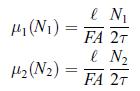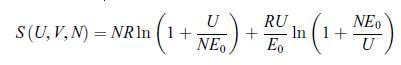Principles Of Thermodynamics 1st Edition Jean-Philippe Ansermet, Sylvain D. Brechet - Solutions
Unlock a world of knowledge with our comprehensive collection of resources for "Principles Of Thermodynamics 1st Edition" by Jean-Philippe Ansermet and Sylvain D. Brechet. Dive into an extensive online answers key and solution manual, where every chapter solution is meticulously detailed. From solved problems and step-by-step answers to the complete instructor manual, our offerings cater to every learning need. Access the test bank, textbook insights, and download solutions in PDF format for free. Enhance your understanding with our expertly crafted questions and answers, ensuring you excel in every aspect of thermodynamics.
![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()