The entropy of a certain fluid has been found to be related to its internal energy and
Question:
The entropy of a certain fluid has been found to be related to its internal energy and volume in the following way:
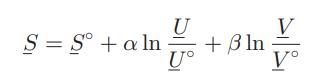
where S°, U°, and V° are, respectively, the molar entropy, internal energy, and volume of the fluid for some appropriately chosen reference state, and α and β are positive constants.
a. Develop an interrelationship between internal energy, temperature, and specific volume (the thermal equation of state) for this fluid.
b. Develop an interrelationship between pressure, temperature, and volume (the volumetric equation of state) for this fluid.
c. Show that this fluid does not have a first-order phase transition by establishing that the fluid is stable in all thermodynamic states.
Step by Step Answer:

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler





