The excess Gibbs energies for liquid argonmethane mixtures have been measured at several temperatures. The results are
Question:
The excess Gibbs energies for liquid argon–methane mixtures have been measured at several temperatures. The results are
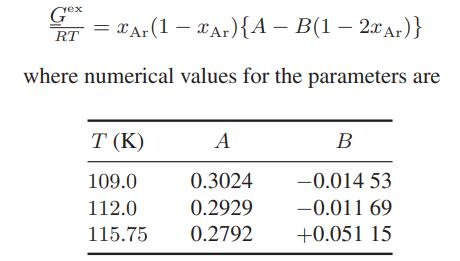
Compute the following:
a. The activity coefficients of argon and methane at 112.0 K and xAr = 0.5
b. The molar isothermal enthalpy change on producing an xAr = 0.5 mixture from its pure components at 112.0 K
c. The molar isothermal entropy change on producing an xAr = 0.5 mixture from its pure components at 112.0 K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





