The extent of reaction generally depends on pressure as well as temperature. For the reaction (or phase
Question:
The extent of reaction generally depends on pressure as well as temperature. For the reaction (or phase transition)
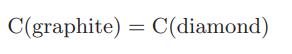
the standard-state Gibbs energy change at 25°C is 2866 J/mol. The density of graphite is 2.25 g/cc and that of diamond is approximately 3.51 g/cc; both solids may be considered to be incompressible. To help chemical engineering students pay their tuition, we are thinking of setting up equipment to run this reaction in the senior laboratory. Estimate what pressure, at room temperature (T ∼ 25°C), must be used to convert old pencil “leads” (graphite) into diamonds.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





