The following data are available for the isothermal heat of mixing of trichloromethane (1) and ethanol (2)
Question:
The following data are available for the isothermal heat of mixing of trichloromethane (1) and ethanol (2) at 30°C [reference: J. P. Shatas, M. M. Abbott, and H. C. Van Ness, J. Chem. Eng. Data, 20, 406 (1975)].
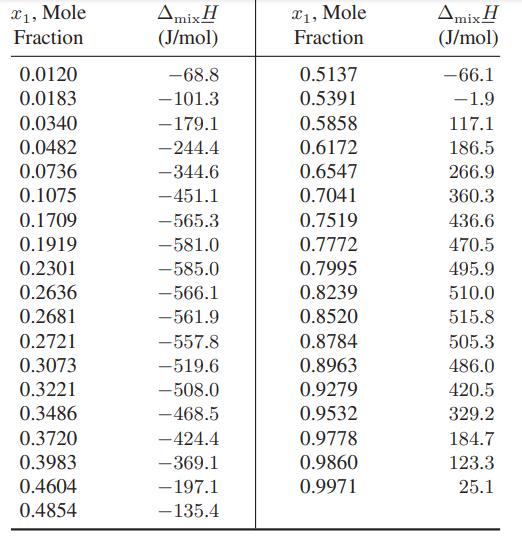
Compute the partial molar enthalpies of trichloromethane and ethanol in their mixtures at 30°C over the whole composition range.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





