An equimolar mixture of nitrogen and acetylene enters a steady-flow reactor at 25C and 1 bar of
Question:
An equimolar mixture of nitrogen and acetylene enters a steady-flow reactor at 25°C and 1 bar of pressure. The only reaction occurring is
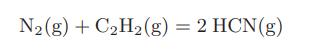
The product leaves the reactor at 600°C and contains 24.2 percent mole fraction of HCN. How much heat is supplied to the reactor per mole of HCN?
Transcribed Image Text:
N₂(g) + C₂H₂(g) = 2 HCN(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To calculate the heat supplied to the reactor per mole of HCN we need to perform an energy balance on the reactor The energy balance states that the t...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
An equimolar mixture of nitrogen and acetylene enters a steady-flow reactor at 25(C and atmospheric pressure. The only reaction occurring is: N2(g) + C2H2 ( 2HCN(g). The product gases leave the...
-
An equimolar mixture of oxygen and nitrogen enters a compressor operating at steady state at 10 bar, 220 K with a mass flow rate (m) of 1 kg/s. The mixture exits the compressor at 60 bar, 400 K with...
-
Jacky is single and will turn 24 this year. He doesn't have any dependents. He does not have any group coverage. He drives a motorcycle to work every day. From your perspective which insurance policy...
-
Pritchett Company reported the following year-end data: Cash $ 25,000 8,000 Short-term investments Accounts receivable (current) Inventory 19,500 27,500 Prepaid (current) assets 11,000 Total current...
-
Yolanda Christophe filed a bankruptcy petition under Chapter 13. Her scheduled debts consist of $11,100 of secured debt, $9,300 owed on an unsecured student loan, and $6,960 of other unsecured debt....
-
(a) Compute the monthly excess returns on the United States stock Exxon and the market excess returns. (b) Compute the variances and covariances of the two excess returns. Interpret the statistics....
-
Two ball bearings, each with 16 balls, are used to support the shaft of a fan that rotates at \(750 \mathrm{rpm}\). Determine the frequencies, in hertz, corresponding to the following defects: *...
-
Multiple-Choice Questions 1. The data attributes that a particular user has permission to access are defined by the a. Operating system view. b. Systems design view. c. Database schema. d. User view....
-
Calculate the current-period financial statement items expressed as a percentage of the base amount for the last two years. In other words, use this relationship: Common-Size Percentage = (comparison...
-
An overnight shipping firm operates major sorting facilities (hubs) in six cities. To compare the performance of the hubs, it tracked the shipping time (in hours) required to process 20 randomly...
-
The following data are available for the isothermal heat of mixing of trichloromethane (1) and ethanol (2) at 30C [reference: J. P. Shatas, M. M. Abbott, and H. C. Van Ness, J. Chem. Eng. Data, 20,...
-
Using the data below, calculate the partial molar enthalpies of 1-propanol and water as a function of composition at both 25C and 50C. data: V. P. Belousov, Vent. Leningrad Univ. Fiz., Khim, 16(1),...
-
Nancy is designing a quilt that she will enter in the quilt competition at the State Fair. The quilt consists of twelve identical squares with 4 rows of 3 squares each. Each square is to have a...
-
Cruse Cleaning offers residential and small office cleaning services. An average cleaning service has the following price and costs. $136.00 per service 98.00 per service Sales price Variable costs...
-
Legacy issues $630,000 of 9.0%, four-year bonds dated January 1, 2021, that pay interest semiannually on June 30 and December 31. They are issued at $571,310 when the market rate is 12%. Required: 1....
-
Solve the equation. 3+p= 7- 32-P V
-
Keisha Tombert, the bookkeeper for Tamarisk Consulting, a political consulting firm, has recently completed a managerial accounting course at her local college. One of the topics covered in the...
-
Chuck, a single taxpayer, earns $79,200 in taxable income and $15,000 in interest from an investment in City of Heflin bonds. (Use the US tax rate schedule.) Required: a. How much federal tax will he...
-
How do increases in unexpected inflation affect P&C insurers?
-
(a) Given a mean free path = 0.4 nm and a mean speed vav = 1.17 105 m/s for the current flow in copper at a temperature of 300 K, calculate the classical value for the resistivity of copper. (b)...
-
Develop a table that lists advantages and disadvantages of the three design concepts for a mousetrap-powered vehicle in the case study in Section 2.4. Discuss the trade-offs between front and rear...
-
Three concepts for the drive mechanism in a mousetrap-powered vehicle are described in Section 2.4. Develop another concept, prepare several sketches, and write a brief description of it.
-
Express your weight in the units of pounds and newtons, and your mass in the units of slugs and kilograms.
-
Let's talk about this. Why would the attorney not be subject to discipline for violating the ethical duty of confidentiality if she provides the testimony without objection? Hi there could you help...
-
Give your opinion/response to the below- Lukis Anderson arrested December 2012, for murder of Raveesh Kumra but actually it was more of framed by his own DNA. His DNA was found under the finger nail...
-
explain the significance of partition coefficients in determining the distribution of solutes between immiscible phases, and how does this concept guide the choice of solvents in extraction processes...

Study smarter with the SolutionInn App


