The Joule-Thomson coefficient, , given by is a function of temperature. The temperature at which =
Question:
The Joule-Thomson coefficient, μ, given by
![= T . || - - -[1 - Ta]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/9/6/4/6836553670be66631699964680686.jpg)
is a function of temperature. The temperature at which μ = 0 is known as the inversion temperature.
a. Use the van der Waals equation of state to determine the inversion temperature of H2, O2, N2, CO and CH4. The van der Waals parameters for these gases can be found in Table 6.4-1.
Table 6.4-1.
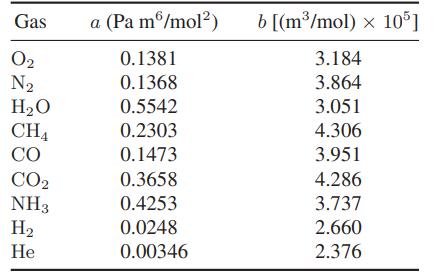
b. Repeat part (a) using the Redlich-Kwong equation of state.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





