Repeat Problem 5.9 assuming that helium is described by the Peng-Robinson equation of state. Problem 5.9 High-pressure
Question:
Repeat Problem 5.9 assuming that helium is described by the Peng-Robinson equation of state.
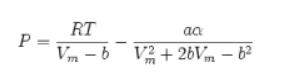
Problem 5.9
High-pressure helium is available from gas producers in 0.045-m3 cylinders at 400 bar and 298 K. Calculate the explosion equivalent of a tank of compressed helium in terms of kilograms of TNT. Assume helium is an ideal gas.
Transcribed Image Text:
P = RT aa Vm-b V2+2bVm – b²
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
To solve this problem we will need to use the PengRobinson equation of state to calculate the fugacity of helium at the given conditions The fugacity ...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
High-pressure helium is available from gas producers in 0.045-m 3 cylinders at 400 bar and 298 K. Calculate the explosion equivalent of a tank of compressed helium in terms of kilograms of TNT....
-
In this problem, we consider the analysis of the combined information from both raters on the shoulder flexion (SF) scores in the posture measurement study. Thus, the questions below concern the data...
-
The molar heat capacity C P,m of SO 2 (g) is described by the following equation over the range 300 K < T < 1700 K: In this equation, T is the absolute temperature in kelvin. The ratios T/K ensure...
-
Suppose you make beautiful coffee tables out of juniper trees. Your materials cost for each table is $135. You pay your craftsman $25 per hour and it takes him 5 hours to complete one table. If you...
-
Several manufacturers introduced into the American market products known as all-terrain vehicles (ATVs). ATVs are motorized bikes that sit on three or four low-pressure balloon tires and are meant to...
-
The absolute entropy for all crystalline substances at absolute zero temperature is (a) Zero (b) Negative (c) More than zero (d) None of these.
-
Anthropologists see a strong correlation between social and religious change. Given the rapid globalization of the international economy, do you believe that religious violence is likely to increase...
-
Develop a chase aggregate plan for Draper using a permanent workforce of 12 employees supplemented by overtime. All demand must be met each period. (a) Show what would happen if this plan were...
-
What is a source that supports one firm raising prices, the other firms will likely follow suit to stay competitive. However, if one firm lowers prices, the other firms will also lower their prices...
-
You are the director of an engineering organization and have been fighting the war for talent for a while. It seems that whenever you have a role vacancy, you let HR know but it takes forever to find...
-
Using the Redlich-Kwong equation of state, compute and plot (on separate graphs) the pressure of nitrogen as a function of specific volume at the two temperatures: a. 110 K b. 150 K
-
The Joule-Thomson coefficient, , given by is a function of temperature. The temperature at which = 0 is known as the inversion temperature. a. Use the van der Waals equation of state to determine...
-
Procter & Gamble has been the leading soap manufacturer in the United States since 1879, when it introduced Ivory soap. However, late in 1991, its major rival, Lever Bros. (Unilever), overtook it by...
-
Calculate the cost of goods sold expense if the company uses the average cost inventory valuation method. Calculate the cost of goods sold expense if the company uses the FIFO inventory valuation...
-
Module: International Trade; Explain giving examples from African countries and support from literature review Explain how does foreign direct investment (FDI) contribute to export growth? What are...
-
Discuss the trend of globalization? What are different modes of economic interrelationships used today in health care? Explain Briefly and Provide a reference
-
A bakery in Champaign , IL is considering the purchase of a $20,000 espresso machine . The coffee maker has an economic life of seven years and will be fully depreciated by the straight - line method...
-
What are some key fixed and variable costs for Acceptance Insurance? Remember, fixed costs do not change when output changes. That is, fixed costs remain even if the company is producing nothing....
-
An insurance company owns $ 50 million of floating-rate bonds yielding LIBOR plus 1 percent. These loans are financed with $ 50 million of fixed-rate guaranteed investment contracts (GICs) costing 10...
-
What are the risks and liability factors in an audit? What are the implications to the auditor? What are the implications to the organization? How can the auditor mitigate these risks and liability...
-
Use a plot over the range 0 x 5 to con rm that sin( ix) = i sinh x.
-
The function y(t) = 1 -! e -b t, where t is time and b > 0, describes many processes, such as the height of liquid in a tank as it is being filled and the temperature of an object being heated....
-
The following functions describe the oscillations in electric circuits and the vibrations of machines and structures. Plot these functions on the same plot. Because they are similar, decide how best...
-
BMX Company has one employee. FICA Social Security taxes are 6.2% of the first $137,700 paid to its employee, and FICA Medicare taxes are 1.45% of gross pay. For BMX, its FUTA taxes are 0.6% and SUTA...
-
An iron boiler of mass 188 kg contains 690 kg of water at 19C. A heater supplies energy at the rate of 58,000 kJh.How long does it take for the water (a) to reach the boiling point, and (b) to all...
-
Skuta Pty Ltd Scenario The scenario provides you with a brief overview of a hypothetical company, 'Skuta' and a contemporary scenario. Be aware that the scenario may not cover every detail that you...

Study smarter with the SolutionInn App


