The sublimation pressure of carbon dioxide as a function of temperature is and the molar volume of
Question:
The sublimation pressure of carbon dioxide as a function of temperature is
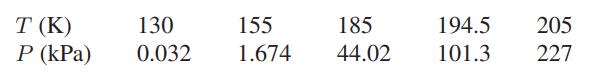
and the molar volume of CO2 is 2.8 × 10−5 m3/mol.
a. Determine the heat of sublimation of CO2 at 190 K.
b. Estimate the fugacity of solid CO2 at 190 K and 200 bar.
Transcribed Image Text:
T (K) P (kPa) 130 0.032 155 1.674 185 44.02 194.5 205 101.3 227
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
Part a To determine the heat of sublimation of CO2 at 190 K we can use the ClausiusClapeyron equatio...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
A student carried out the following procedure to measure the pressure of carbon dioxide in a soft drink bottle. First, she weighed the bottle (853.5 g). Next, she carefully removed the cap to let the...
-
The atmosphere of the planet Venus is almost entirely composed of carbon dioxide (about 96.5 % carbon dioxide). The carbon dioxide on Venus might be in equilibrium with carbonate ions in minerals on...
-
In Exercises identify the open intervals on which the function is increasing or decreasing. f(x) = sinx + sin x, 0 < x < 2
-
What does traceability mean?
-
Determine the angular momentum HO of each of the two particles about point O. use a scalar solution. 15 m/s 4 m 2 kg 1.5 m 2 m 4 m 300 | B 1.5 ke 10 m/s m ITI
-
For the reaction \(2 \mathrm{SO}_{2}+\mathrm{O}_{2} \Leftrightarrow 2 \mathrm{SO}_{3}, \Delta H=-42 \mathrm{kcal}\), the forward reaction will be favoured by (a) Low temperature (b) High pressure (c)...
-
In Problems 5-15 and 5-16, three different forecasts were developed for the demand for fertilizer. These three forecasts are a 3-year moving average, a weighted moving average, and a trend line....
-
List the major regions of the EM spectrum from shortest wavelength to longest wavelength. For the visible part of the spectrum, list the shortest and longest wavelengths of visible light. For each...
-
Use the Analysis>Pure tool in Aspen Plus with the Peng-Robinson equation of state to compute the vapor pressures of the refrigerants R12 (dichlorodifluoromethane, or Freon 12), R124...
-
Redo Illustration 7.5-2 using the SoaveRedlichKwong equation of state. Illustration 7.5-2 Complete the calculated thermodynamic properties chart for oxygen by considering temperatures between 100C...
-
Management is exploring the possibility of adding more agents at the check-in counter of an airline to reduce the waiting time of customers. Based on available information, the standard deviation of...
-
A chemical plant produces sodium bisulfate in 100 kg bags. Demand for this product is 28 tonnes per day. The capacity for producing this product is 60 tonnes per day. Setup cost is $420, and holding...
-
Choi Company manufactures two skin care lotions, Smooth Skin and Silken Skin, from a joint process. The joint costs incurred are $430,000 for a standard production run that generates 200,000 pints of...
-
Steinberg Company had the following direct materials costs for the manufacturing of product T in March: Actual purchase price per pound of direct materials $ 9.00 Standard direct materials allowed...
-
The following data from the just completed year are taken from the accounting records of Mason Company: Sales $ 656,000 Direct labor cost $ 83,000 Raw material purchases $ 132,000 Selling expenses $...
-
California Surf Clothing Company issues 1,000 shares of $1 par value common stock at $35 per share. Later in the year, the company decides to purchase 100 shares at a cost of $38 per share. Assume...
-
Discuss an auditors objectives in the audit of equity accounts. Describe appropriate analytical procedures an auditor may apply to equity accounts.
-
Avatar Financials, Inc., located on Madison Avenue, New York City, is a company that provides financial advice to individuals and small- to mid-sized businesses. Its primary operations are in wealth...
-
The bearing consists of rollers, symmetrically confined within the housing. The bottom one is subjected to a 125-N force at its contact A due to the load on the shaft. Determine the normal reactions...
-
The members of a truss are connected to the gusset plate. If the forces are concurrent at point O, determine the magnitudes of F and T for equilibrium.Take = 90. 9 kN F S03 r000 1006
-
The man attempts to pull down the tree using the cable and small pulley arrangement shown. If the tension in AB is 60 lb, determine the tension in cable CAD and the angle which the cable makes at...
-
V+w=1x2 Vyz 1. Solve an equation for any variable. Solve the following for x. a) v+w= xyz 2 11 b) ==+- x 3 2 vt x yz b x 21 y d) 32 2. Be able to reduce fractions containing powers of ten. 102 a)...
-
By making Section 5 the heart of the securities regulation framework, the regime's framers embraced a system that is more concerned with the quality of disclosure than the underlying merits of any...
-
Kelly Brown and Bradley Brown are recent newlyweds and they have decided to purchase their first home together from Kevin Smith and Karen Smith for $635,000.00 (HST included). Kelly and Bradley's...

Study smarter with the SolutionInn App


