(12.0 mathrm{kmole}) of a mixture (40 mathrm{~mol} %) water and (60 mathrm{~mol} % mathrm{n})-butanol at 1.0 atm...
Question:
\(12.0 \mathrm{kmole}\) of a mixture \(40 \mathrm{~mol} \%\) water and \(60 \mathrm{~mol} \% \mathrm{n}\)-butanol at 1.0 atm pressure is batch distilled in a system with an equilibrium still pot and a column with one equilibrium stage (total two equilibrium contacts). Vapor from the column is sent to a total condenser and then to a liquid-liquid settler. The aqueous phase from the settler \((97.5 \mathrm{~mol} \%\) water) is removed as the distillate product. The organic phase from the settler ( \(57.3 \mathrm{~mol} \%\) water) is refluxed to the distillation column. (The equipment is a modification of Figure 9-10 with a column between the still pot and the condenser.) \(\mathrm{D}_{\text {total }}=3.9 \mathrm{kmol}\). Equilibrium data are in Table 8-5 (in Problem 8.D3).
a. Find \(\mathrm{W}_{\text {final }}\) and \(\mathrm{x}_{\mathrm{W}, \text { fin }}\).
b. Find the approximate value of the reflux ratio L/D at end of the batch distillation.
Figure 9-10
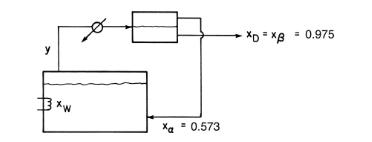
Table 8-5
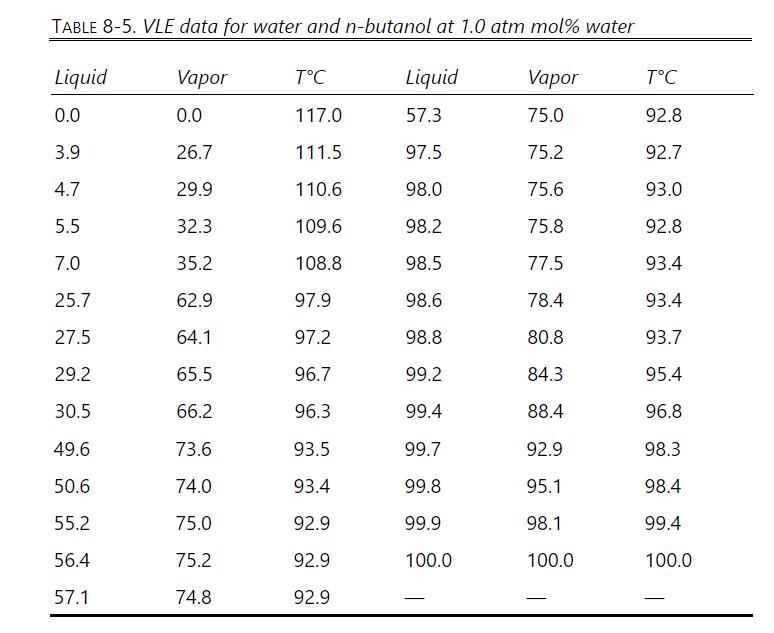
Problem 8.D3
VLE data for water and n-butanol are given in Table 8-5. We have flash distillation systems separating \(100.0 \mathrm{kmol} / \mathrm{h}\) of two different water and \(\mathrm{n}-\) butanol mixtures.
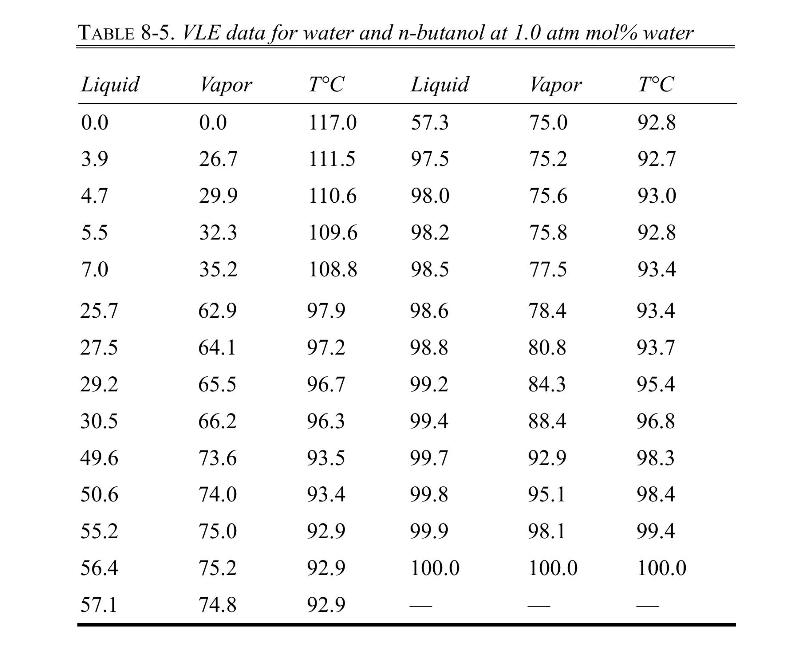
a. The feed is \(20.0 \mathrm{~mol} \%\) water, and the vapor product is \(40.0 \mathrm{~mol} \%\) water. Find \(\mathrm{L}, \mathrm{x}, \mathrm{V}\), and \(\mathrm{T}_{\text {drum. }}\).
b. The feed is \(99.0 \mathrm{~mol} \%\) water, and \(30.0 \%\) of the feed is vaporized. Find \(\mathrm{L}, \mathrm{x}, \mathrm{V}, \mathrm{y}\), and \(\mathrm{T}_{\text {drum }}\).
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





