a. 10 L each of ethanol at 1.1C (30F), ethylene glycol at 6.67C (20F), and water at
Question:
a. 10 L each of ethanol at –1.1◦C (30◦F), ethylene glycol at –6.67◦C (20◦F), and water at 26.7◦C (80◦F) are poured into a tank and mixed. What is the final temperature? Relevant physical property data are 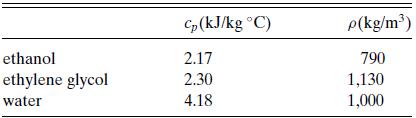
b. The final temperature must be 21.1◦C (70◦F). Only the inlet temperature of the water can be adjusted. What should the water temperature be?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





