Question: A continuous column flash system (Figure 4-24) is separating (100 mathrm{kmol} / mathrm{h}) of a saturated liquid feed that is (45 mathrm{~mol} %) methanol and
A continuous column flash system (Figure 4-24) is separating \(100 \mathrm{kmol} / \mathrm{h}\) of a saturated liquid feed that is \(45 \mathrm{~mol} \%\) methanol and \(55 \mathrm{~mol} \%\) water at \(1.0 \mathrm{~atm}\). Operate with \(\mathrm{L} / \mathrm{V}=1.5\) and the outlet bottoms at \(\mathrm{x}_{\mathrm{N}}=0.28\). Data are in Table 2-1 and Problem 3.E1.
a. Find the values of \(\mathrm{F}_{\mathrm{L}}, \mathrm{F}_{\mathrm{V}}, \mathrm{y}_{1}\), and the number of equilibrium stages required.
b. Find the value of \(\mathrm{Q}\) used to vaporize \(\mathrm{F}_{\mathrm{V}}\).
c. For a normal flash with the same feed and the same V/F, find the values of \(\mathrm{x}\) and \(\mathrm{y}\).
Problem 3.E1.

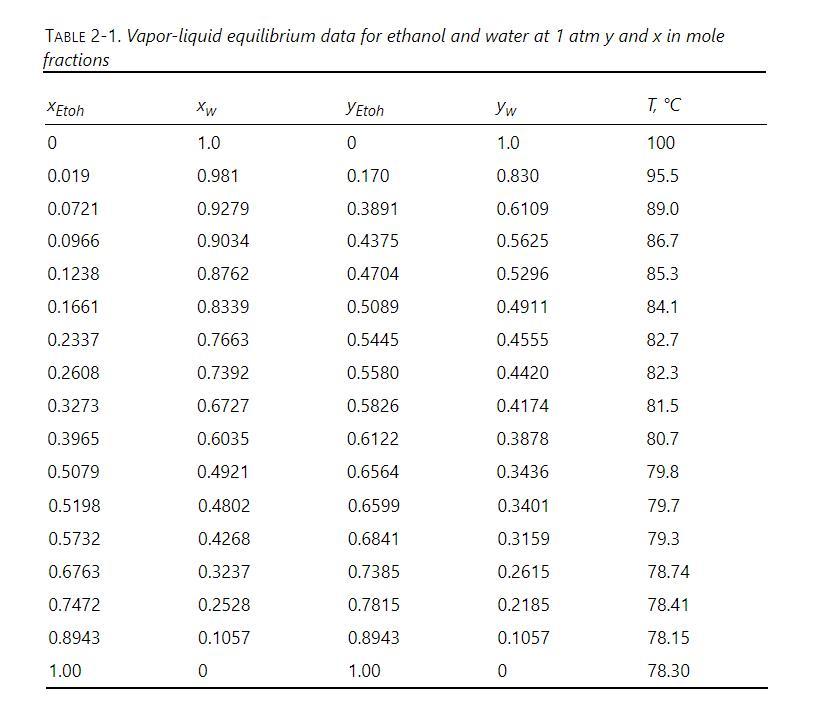
TABLE 2-1. Vapor-liquid equilibrium data for ethanol and water at 1 atm y and x in mole fractions XEtoh Xw YEtoh Yw T, C 0 1.0 0 1.0 100 0.019 0.981 0.170 0.830 95.5 0.0721 0.9279 0.3891 0.6109 89.0 0.0966 0.9034 0.4375 0.5625 86.7 0.1238 0.8762 0.4704 0.5296 85.3 0.1661 0.8339 0.5089 0.4911 84.1 0.2337 0.7663 0.5445 0.4555 82.7 0.2608 0.7392 0.5580 0.4420 82.3 0.3273 0.6727 0.5826 0.4174 81.5 0.3965 0.6035 0.6122 0.3878 80.7 0.5079 0.4921 0.6564 0.3436 79.8 0.5198 0.4802 0.6599 0.3401 79.7 0.5732 0.4268 0.6841 0.3159 79.3 0.6763 0.3237 0.7385 0.2615 78.74 0.7472 0.2528 0.7815 0.2185 78.41 0.8943 0.1057 0.8943 0.1057 78.15 1.00 0 1.00 0 78.30
Step by Step Solution
3.28 Rating (148 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


