Question: A distillation column operating at (1.0 mathrm{~atm}) is separating a mixture of methanol and water. The feed is a saturated liquid. The column has a
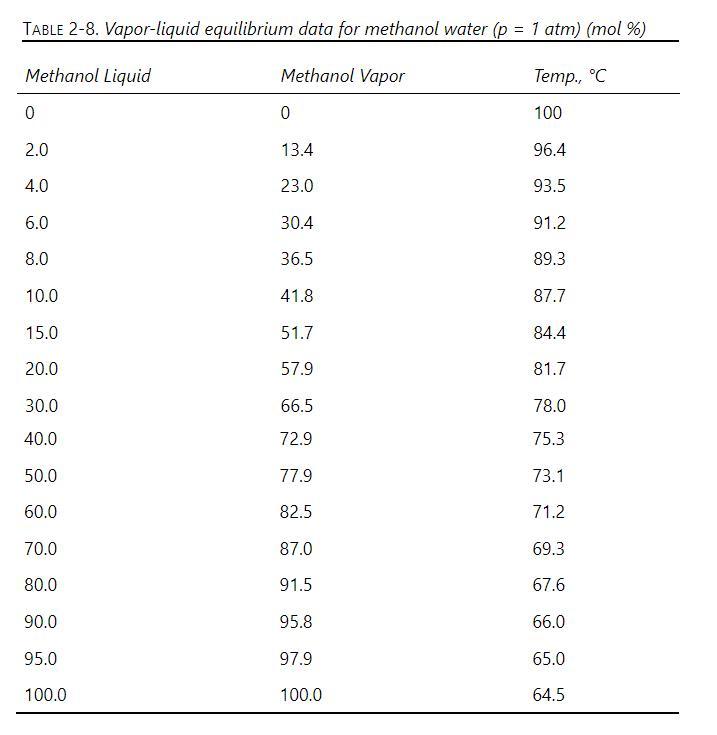
A distillation column operating at \(1.0 \mathrm{~atm}\) is separating a mixture of methanol and water. The feed is a saturated liquid. The column has a total condenser and a partial reboiler. The reflux is returned as a saturated liquid, and constant molal overflow is assumed to be valid. Feed flow rate is \(50 \mathrm{kmol} / \mathrm{h}\). We desire a bottoms composition of \(5 \mathrm{~mol} \%\) methanol and a distillate composition of \(90 \mathrm{~mol} \%\) methanol. The reflux ratio is \(\mathrm{L} / \mathrm{D}=1\), and the boilup ratio is \(v / \mathrm{B}=2\). VLE data are in Table 2-8.
a. Derive and plot the operating lines.
b. Starting at the top of the column, determine the optimum feed stage and the total number of equilibrium stages needed.
c. Determine the feed mole fraction methanol.
TABLE 2-8. Vapor-liquid equilibrium data for methanol water (p = 1 atm) (mol %) Methanol Liquid Methanol Vapor Temp., C 0 0 100 2.0 13.4 96.4 4.0 23.0 93.5 6.0 30.4 91.2 8.0 36.5 89.3 10.0 41.8 87.7 15.0 51.7 84.4 20.0 57.9 81.7 30.0 66.5 78.0 40.0 72.9 75.3 50.0 77.9 73.1 60.0 82.5 71.2 70.0 87.0 69.3 80.0 91.5 67.6 90.0 95.8 66.0 95.0 97.9 65.0 100.0 100.0 64.5
Step by Step Solution
3.50 Rating (147 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


