Question: A simple batch still is separating a feed that is (60.0 mathrm{~mol} % 1,2) dichloroethane and (40.0 mathrm{~mol} %) 1,1,2-trichloroethane. Pressure is (1 mathrm{~atm}). The
A simple batch still is separating a feed that is \(60.0 \mathrm{~mol} \% 1,2\) dichloroethane and \(40.0 \mathrm{~mol} \%\) 1,1,2-trichloroethane. Pressure is \(1 \mathrm{~atm}\). The relative volatility is constant, \(\alpha_{\mathrm{di}-\mathrm{tri}}=2.4\). The use of Eq. \((9-13)\) is recommended.
Equation 9-13
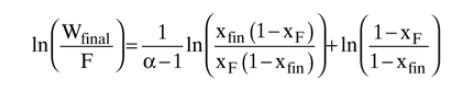
a. The charge \((\mathrm{F})\) is \(1.3 \mathrm{kmol}\). The final still pot concentration is 30.0 \(\mathrm{mol} \% 1,2\) dichloroethane. Find the final moles in the still pot and the average mole fraction of distillate product.
b. Repeat part a if \(\mathrm{F}=3.5 \mathrm{kmol}\).
c. If \(\mathrm{F}=2.0 \mathrm{kmol}\) and the average distillate concentration is \(75.0 \mathrm{~mol} \%\) 1,2-dicholoroethane, find final kmoles in the still pot and the final mole fraction of 1,2 dichloroethane in the still pot.
Wfinal Xfin (1-XF) 1-XF In -In +In F -1 XF (1-xfin), 1-Xfin
Step by Step Solution
3.36 Rating (152 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


