Question: Develop a spreadsheet to solve problem 2.D13, parts b and c. Use Eq. (2-28). (Note: The easiest approach to solving this problem is to develop
Develop a spreadsheet to solve problem 2.D13, parts b and c. Use Eq. (2-28). (Note: The easiest approach to solving this problem is to develop a spreadsheet similar to Figures 2-B2 and run the spreadsheet first for part b and next for part c.)
Problem 2.D13
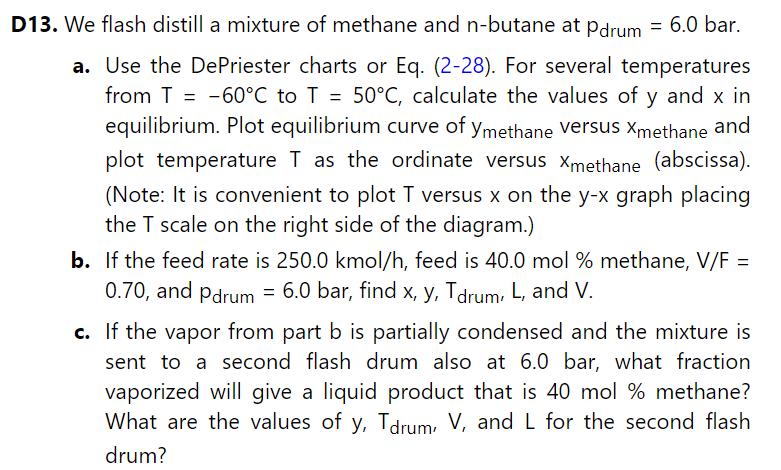
Equation 2-28
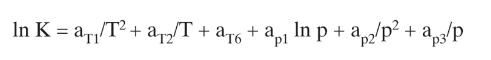
Figure 2-B2
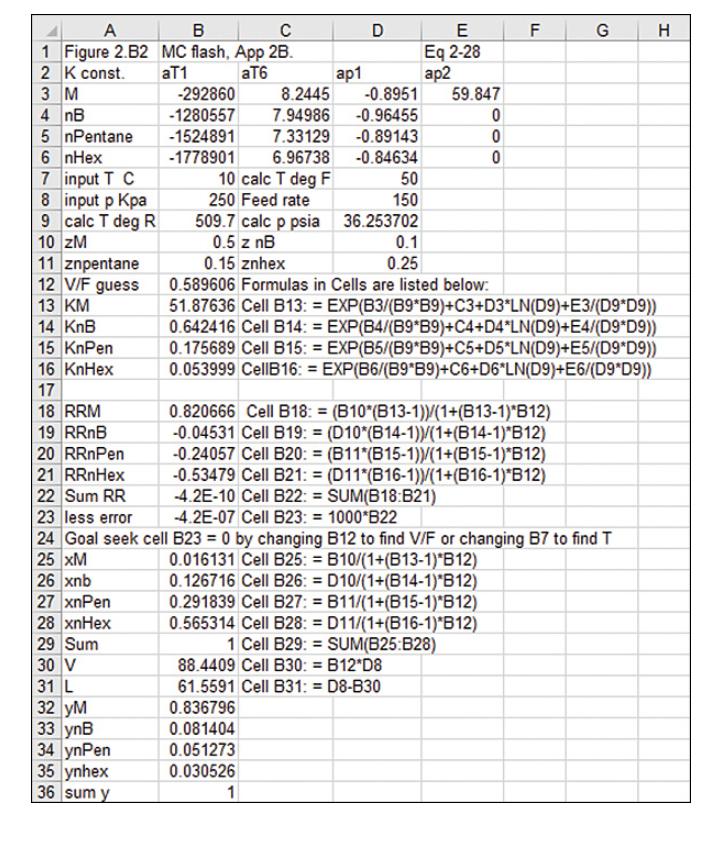
D13. We flash distill a mixture of methane and n-butane at Pdrum = 6.0 bar. a. Use the DePriester charts or Eq. (2-28). For several temperatures from T-60C to T = 50C, calculate the values of y and x in equilibrium. Plot equilibrium curve of ymethane versus Xmethane and plot temperature T as the ordinate versus Xmethane (abscissa). (Note: It is convenient to plot T versus x on the y-x graph placing the T scale on the right side of the diagram.) b. If the feed rate is 250.0 kmol/h, feed is 40.0 mol % methane, V/F = 0.70, and Pdrum = 6.0 bar, find x, y, Tdrum, L, and V. c. If the vapor from part b is partially condensed and the mixture is sent to a second flash drum also at 6.0 bar, what fraction vaporized will give a liquid product that is 40 mol % methane? What are the values of y, Tdrum, V, and L for the second flash drum?
Step by Step Solution
3.50 Rating (150 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


