Find the heat of reaction H R for each of the following reactions using both heat of
Question:
Find the heat of reaction ΔHR for each of the following reactions using both heat of formation and heat of combustion data, as available.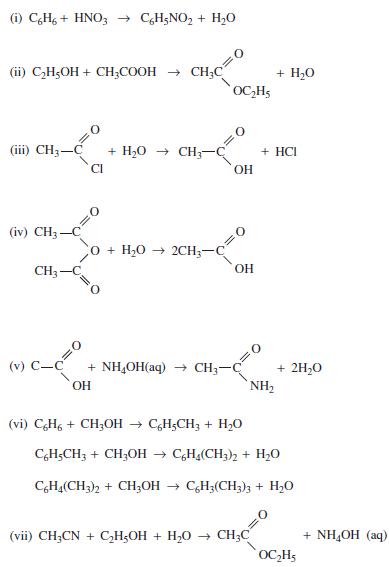
Transcribed Image Text:
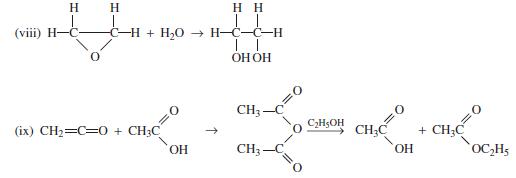
(1) C6H6+ HNO3 → C6H5NO₂ + H₂O (ii) C₂H5OH + CH₂COOH → CH₂C (iii) CH3-C + H,O → CH CI (iv) CH3-C CH3- (v) C-C O + H,O → 2CH + NH₂OH(aq) → CH₂- OH OC₂H₂ OH OH (vi) C&H6 + CH3OH → C6H₂CH3 + H₂O NH₂ + H₂O + HCI (vii) CH3CN + C₂H5OH + H₂O → CH3C + 2H₂O C6H5CH3 + CH₂OH→ C6H4(CH3)2 + H₂O C6H4(CH3)2 + CH3OH → C6H3(CH3)3 + H₂O OC₂H5 + NH,OH (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
To calculate the heat of reaction HR for each chemical reaction you can use either the heats of form...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
a. Derive an equation for the heat of reaction at any temperature if you are given the heat of reaction H R R(T o ) at a standard temperature T o . You may assume an ideal solution. b. Find the...
-
Ethyl alcohol (ethanol) can be produced by the fermentation of sugars derived from agricultural products such as sugarcane and corn. Some countries without large petroleum and natural gas...
-
Ethyl alcohol (ethanol) can be produced by the fermentation of sugars derived from trains and other agricultural products. Some countries without large petroleum and natural as reservessuch as...
-
Translate the seven steps to Java code.
-
Explain why inherent risk is set for segments rather than for the overall audit. What is the effect on the amount of evidence the auditor must accumulate when inherent risk is increased from medium...
-
A skater with an initial speed of 7.60 m/s stops propelling himself and begins to coast across the ice, eventually coming to rest. Air resistance is negligible. (a) The coefficient of kinetic...
-
Alternate Coding Schemes for the Regression Approach to Analysis of Variance. Consider Eq. (8.18), which represents the regression model corresponding to an analysis of variance with three treatments...
-
Recreational Supplies Co. has net sales of $11,655,000, an ROE of 17.64 percent, and a total asset turnover of 2.89 times. If the firm has a debt-to-equity ratio of 1.43, what is the companys net...
-
4. A 57-kg person is on top of a 2.1-kg skateboard and holds a 1.7-kg bowling ball. Initially the skateboard and the person are at rest. The person now throws the bowling bowl 18 m/s East. (A) Which...
-
a. 5 kg/hr of sodium hydroxide and 10 kg/hr of water are to be mixed in an insulated tank. Both feed streams are to be put into the mixer at the same temperature. What is a safe inlet temperature at...
-
A continuous mixing operation is carried out by adding 0.3 kg/hr of calcium chloride crystals to a pure water stream flowing at 1 kg/hour. a. If both streams are at 25C when they enter the mixer,...
-
Green Pastures is a 400-acre farm on the outskirts of the Kentucky Bluegrass, specializing in the boarding of broodmares and their foals. A recent economic downturn in the thoroughbred industry has...
-
Under final Capitalization and Repairs regulations, a self-employed taxpayer may elect to apply a de minimis safe harbor to amounts he or she paid to acquire or produce tangible property to the...
-
do FFIEC Exam procedures call for different testing procedures depending on whether you are a Bank or a Credit Union?
-
Do you think recognizing revenue too early had anything to do with the gyms closing down?
-
What is the purpose of the Payment Bond? What percentage of the project cost should be covered by the Payment Bond? What should be bond date? What is the owner obligated to do if it receives any...
-
Provide in detail how you analyse and interpret budget and actual financial information to monitor and review profit and productivity performance?
-
Perry Chandler, a broker with Caveat Emptor, Ltd., offers free investment seminars to local PTA groups. On average, Chandler expects 1 percent of seminar participants to purchase $25,000 in...
-
Let (X. A. p) be a measure space. Show that for any A,B A, we have the equality: (AUB)+(An B) = (A) + (B).
-
If the sucrose concentration is 20% what will be the flow rate at the higher pressure in the previous problem.
-
A solution of sucrose in water is concentrated by RO. It is found that, with a differential applied pressure of 6000 kPa, the rate of movement of the water molecules through the membrane is 25 kg m2...
-
Derive the equation for Langmuir adsorption isotherm.
-
Reflective essay on international business trends. It is important that social, political, legal, cultural factors, production factors and their movement across borders, globalization, government...
-
How do you determine an employee's emotional stability to things like stress in the workplace? Is this ability learned by you do you think it is something you were born with?
-
How well did Allen interact with each member of the buying center? (Lawford Electric case) Lawford Electric Company (Revised) 580-124 Exhibit 1 (continued) Tension Reel-Hallden Shear System consists...

Study smarter with the SolutionInn App


